Summary
DOI: 10.1373/clinchem.2017.283507
Student Discussion
Student Discussion Document (pdf)
Alicia Hutcherson Wright,1 Jeffrey A. Young,1 Breland Elise Smith,2 and Alec Saitman1*
1Providence Regional Laboratories, Providence, OR; 2InSource Diagnostics, Monrovia, CA
*Address correspondence to this author at: Providence Regional Laboratories, 4400 NE Halsey Street Building 3, Portland, OR 97213. E-mail [email protected]
Case Description
A 31-year-old pregnant woman at 25-week gestation was admitted for preterm premature rupture of membranes and subsequently delivered preterm at 29 weeks. The patient insisted that she was unaware of the pregnancy and, thus, received no prenatal care. She had a clinically significant polysubstance abuse history: intravenous heroin, prescription amphetamine (Adderall), methamphetamine, tobacco, marijuana, and alcohol. There was also history of anxiety and postpartum depression following prior pregnancies. During her hospital stay, she entered into a
medication-assisted treatment program and was prescribed buprenorphine (Subutex) for opiate addiction and fluoxetine (Prozac) for depression. On discharge, she entered a postpartum outpatient program for addiction treatment that required regular urine drug screen submissions with the
goal of abstinence.
During an initial urine drug screen submission after admission into the treatment program, an immunoassay drug screen was presumptively positive for the amphetamines class with a cutoff of 300 ng/mL and negative for all other components. Confirmation by an inhouse liquid
chromatography–tandem mass spectrometry (LC-MS/MS) assay yielded a methamphetamine measurement of 4990 ng/mL and undetectable concentrations of amphetamine with a limit of detection of 100 ng/mL (positive cutoff of 200 ng/mL). This atypical metabolite pattern was observed in subsequent drug screen/confirmation algorithms 2 weeks, 3 weeks, and 3
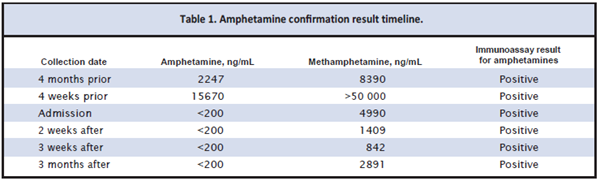
months after the initial occurrence. Chart review of previous methamphetamine screens and confirmations revealed typical metabolic patterns of high methamphetamine concentrations with detectable amphetamine presence (Table 1).
Questions to Consider
- How should metabolite patterns be incorporated in the clinical interpretation of drug confirmation results?
- Should specific concentrations of metabolites be present to report a positive methamphetamine concentration in a clinical drug testing setting?
- Why should medication lists be used in the clinical interpretation of drug confirmation results?
- What additional testing should be requested for the patient in this case?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the September 2018 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2017.283507
Copyright © 2018 American Association for Clinical Chemistry