The causal role of low-density lipoprotein-cholesterol (LDL-C) in the development of ASCVD has been well demonstrated by genetic, observational, and interventional studies (1). That is why the primary therapeutic target for ASCVD risk reduction is to decrease concentrations of LDL-C, a strategy endorsed by multiple US (2) and European (3) guidelines. Yet, despite intensive LDL-lowering therapies, a significant residual ASCVD risk persists, particularly for patients with elevated triglyceride (TG) concentrations (4).
LDL-TG was first reported to be an independent predictor of ASCVD events and superior to LDL-C in the Atherosclerosis Risk in Communities (ARIC) study (5). Recently, in two large prospective cohorts from a large European population study, it was reported that elevated concentrations of LDL-TG are strongly associated with an increased risk of ASCVD, which was confirmed with additional meta-analyses of previous studies (6). Moreover, a Bayesian network analysis of the potential coronary atherosclerotic biomarkers in the GLOBAL clinical study confirmed that LDL-TG was possibly directly linked to the pathogenesis of atherosclerosis (7).
Although a direct assay for LDL-TG has been reported (8), it is not yet approved by the FDA. We, therefore, investigated whether an equation could be developed for LDL-TG based on the standard lipid panel results similarly to what is routinely done for estimating LDL-C and whether it could be used as a univariate ASCVD risk marker.
In order to develop an equation for estimated LDL-TG (eLDL-TG), we used standard lipid data from a general dyslipidemic population, which were also analyzed by the beta-quantification (BQ) reference method for both cholesterol and TG in various fractions. The newly developed equation is as follows:
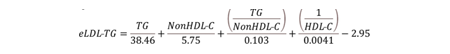
The comparison between LDL-TG calculated with the eLDL-TG equation and BQ-measured LDL-TG was only modest (R2=0.601, slope=0.611), but eLDL-TG showed better ASCVD risk stratification than LDL-C calculated by the NIH-Sampson or other LDL-C equation (9) by survival curve analysis in ARIC and UK Biobank datasets. Moreover, receiver-operating characteristic (ROC) analysis showed that eLDL-TG as a univariate risk marker was the best predictor of ASCVD events than all other traditional lipid-marker tests for ASCVD events. In the National Health and Nutrition Examination Survey (NHANES) database, which is representative of the general US population, eLDL-TG was more strongly associated with hypertriglyceridemia, obesity, diabetes, metabolic syndrome and increased high-sensitivity C-reactive protein than NIH-Sampson LDL-C. Inclusion of eLDL-TG in a Cox-Proportional Hazards Model, along with other conventional risk factors (age, sex, race, diabetes, systolic blood pressure, smoking, HDL-C, and total cholesterol), however, did not further improve ASCVD risk prediction.
One possible reason for the better performance of LDL-TG as an univariate risk marker over LDL-C is that as can be seen by the equation it is positively related to TG, whereas LDL-C tends to decrease with increasing TG by both the calculation and when directly measured. More studies, however, will be needed to understand how LDL-TG is linked to the pathogenesis of ASCVD and how to best integrate this new biomarker into current guidelines.
References
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel [eng]. Eur Heart J 2017 Aug 21;38 32:2459-72 as doi: 10.1093/eurheartj/ehx144.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139 25:e1082-e143 as doi: doi:10.1161/CIR.0000000000000625.
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice [eng]. Eur Heart J 2021 Sep 7;42 34:3227-337 as doi: 10.1093/eurheartj/ehab484.
- Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management [eng]. J Am Coll Cardiol 2018 Jul 17;72 3:330-43. Epub 20180620 as doi: 10.1016/j.jacc.2018.04.061.
- Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, et al. Remnant-Like Particle Cholesterol, Low-Density Lipoprotein Triglycerides, and Incident Cardiovascular Disease [eng]. J Am Coll Cardiol 2018 Jul 10;72 2:156-69 as doi: 10.1016/j.jacc.2018.04.050.
- Balling M, Afzal S, Davey Smith G, Varbo A, Langsted A, Kamstrup PR, Nordestgaard BG. Elevated LDL Triglycerides and Atherosclerotic Risk. Journal of the American College of Cardiology 2023 2023/01/17/;81 2:136-52 as doi: https://doi.org/10.1016/j.jacc.2022.10.019.
- Voros S, Bansal AT, Barnes MR, Narula J, Maurovich-Horvat P, Vazquez G, Marvasty IB, et al. Bayesian network analysis of panomic biological big data identifies the importance of triglyceride-rich LDL in atherosclerosis development [eng]. Front Cardiovasc Med 2022;9:960419. Epub 20230104 as doi: 10.3389/fcvm.2022.960419.
- Ito Y, Ohta M, Ikezaki H, Hirao Y, Machida A, Schaefer EJ, Furusyo N. Development and Population Results of a Fully Automated Homogeneous Assay for LDL Triglyceride [eng]. J Appl Lab Med 2018 Mar 1;2 5:746-56 as doi: 10.1373/jalm.2017.024554.
- Sampson M, Ling C, Sun Q, Harb R, Ashmaig M, Warnick R, Sethi A, et al. A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients With Normolipidemia and/or Hypertriglyceridemia [eng]. JAMA Cardiol 2020 May 1;5 5:540-8 as doi: 10.1001/jamacardio.2020.0013.
Affiliations
- Lipoprotein Metabolism Laboratory, Translational Vascular Medicine Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA
- Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD, USA
To whom correspondence should be addressed
Anna Wolska
Lipoprotein Metabolism Laboratory, Translational Vascular Medicine Branch
National Heart, Lung, and Blood Institute, National Institutes of Health
9000 Rockville Pike
Bldg. 10/Rm. 5D15
Bethesda, MD 20892
Tel: 301-496-3707
Fax: 301-402-1885
e-mail: [email protected]
Acknowledgement
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI) at National Institutes of Health.