As laboratory professionals, it is not uncommon for us to find ourselves at odds with our industry/vendor partners. Since I began my training in clinical chemistry, there always seemed to be an unspoken mentality (fallacy) that individuals/organizations operating under a for-profit structure could not possibly prioritize patient safety and/or quality laboratory testing over revenue. In Canada, where healthcare is (mostly) covered under universal health insurance, there is an even greater chasm at times between the clinical laboratory and vendors because of this perceived difference in philosophy. In spite of this, I have grown to not only appreciate the mutual benefits of developing a strong relationship with vendors but also see it as a necessary aspect to truly deliver high-quality laboratory services.
Within the past few years, I have come to learn that our vendor partners are truly masters of their craft and work within a unique scientific-industry interface and a narrow regulatory framework that we as laboratorians are not privy to. In the same way that we laboratorians are constantly searching for ways to translate laboratory knowledge to clinicians and patients, our vendor colleagues are constantly searching for ways to translate our feedback to their executives (who may or may not have any scientific background) to drive change within their organization. In addition to the familiar roles of account executives, marketing professionals, engineers and field application specialists, there is a diverse network of multi-disciplinary professionals on the vendor side who constantly strive for development and product improvement nurtured by the stiff competition within the in vitro diagnostics (IVD) industry.
These include, but are not limited to: clinical laboratory scientists who may be involved in marketing, sales, technical service, customer care or manufacturing, and board-certified clinical chemists working in assay development, medical or scientific affairs or are involved in clinical trial design. It would be remiss of us to not acknowledge the vast education and training that our vendor colleagues undergo to be able to fulfill all of these roles. Needless to say, building collaborative relationships with such vendor colleagues can add tremendous value to your laboratory beyond the usual service needs such as consultation on workflow analysis, information on products in the pipeline and leveraging purchasing power, to name a few.1 Other opportunities for productive industry partnerships lie in advisory roles, collaborative studies and clinical studies for the development of new products and novel intended uses.
At the same time, it is in the best interest of vendors to forge and maintain strong relationships with their laboratory clients. Amongst the various reasons for vendors to invest in their customers, the most compelling one – at least from my perspective – is that laboratories can provide direct feedback and insight on the performance of products in “live” environments. There are a host of factors that vendors are unable to simulate during pre-market evaluation of IVD products, especially for high-volume, automated systems. The impact of such factors – which can include environmental conditions, institutional/laboratory-specific configurations, and human factors – on performance often do not manifest until the product (hardware, software and assay) hits the market. As such, laboratory customers are a wealth of feedback and direct experience related to IVD products’ usability, performance and overall quality with respect to clinical testing. All of this information can be used by vendors to guide and direct continuous improvement of their IVD products, which will always be beneficial for both vendors and laboratories. Furthermore, clinical laboratory directors support the diagnostics industry as key opinion leaders or clinical study sites. Having said this, laboratories will only feel obliged to provide such information if there is tacit trust that their vendor colleagues are not only receptive to such feedback, but also act accordingly within their roles to drive improvements and positive changes. Conversely, vendors that adopt defensive and resistant attitudes towards clients will likely never be privy to this essential information.
In general, there are still gaps in our training programs for young laboratorians on exposure and management of the vendor aspect of laboratory testing. Given the numerous benefits of forging strong vendor relationships, it is critical that we engage our trainees in interacting with our vendor partners as early as possible and intentionally throughout training programs. The Siemens Healthineers Medical & Scientific Learning Exchange Program offers a unique path for young laboratorians to gain insights into the highly regulated environment of in vitro diagnostics. This onsite program offers young laboratorians a vendor agnostic view of the product development lifecycle, regulatory obligations for IVD manufacturers and the complaint management process. 2 Other opportunities to interact with vendors include the annual AACC Meeting Expo where the majority of key IVD vendors can be found all in one location.
So how does one go about building relationships with vendor colleagues? The answer is simple - in the same manner that you would build any productive relationship in your life, whether professional or personal. Forming and shaping a relationship takes time, patience and effort as both parties need to be convinced that it is a worthwhile endeavour throughout successive “milestones”. A common framework to describe these milestones is the Knapp Relational Development Model:
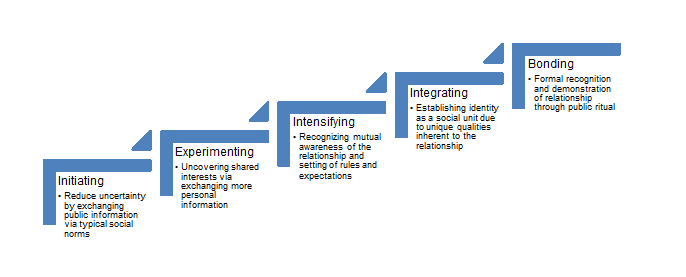
Figure 1. Adaptation of Knapp’s Relational Model (Source: http://stuartmcneil.blogspot.com/2017/10/knapps-relational-model.html).
While every relationship does not necessarily progress in this sequence, it is crucial to recognize that a successful and productive relationship requires mutual confidence from both parties that these milestones have been achieved at some point in time. As with any relationship, attaining the final milestone of bonding may require years and even then, still requires conscious thought and effort from both parties consistently as relationships can stagnate over time.
A final, and important, reflection is that even the best relationships built over many years can be broken at a whim. A common error that I have observed in laboratory-vendor relationships (which I myself have been guilty of as well) is overlooking mutual respect and trust when mistakes occur – a scenario that is all too familiar would be when a perceived instrumentation error has led to complaints about inaccurate results. In such cases, it is imperative that both parties exercise respect, empathy and constructive communication as it only takes one insensitive comment or disinterested gesture to undo all of the time and effort in establishing the relationship. For me, the best reminders in these situations are: (1) It is always beneficial to the laboratory for vendor colleagues to feel that it is within their best interests to have a relationship with us; and (2) Adversity and conflict are always opportunities to further strengthen a relationship.
References
1. Holshoe, K. (2019, September 19). The Value Of Strong Vendor Relationships. https://staffready.com/article/Value-stong-vendor-relationships
2. https://www.siemens-healthineers.com/en-us/education/personalized-education-by-solution/solution/lab-learning-exchange-program (Accessed January 20, 2023).