It is well recognized that a majority of lab testing errors occur in the preanalytical phase prior to the blood sample being placed onto the laboratory analyzer (1). The frequency at which such errors have been reported is summarized in the following figure adapted from multiple sources (2-6).
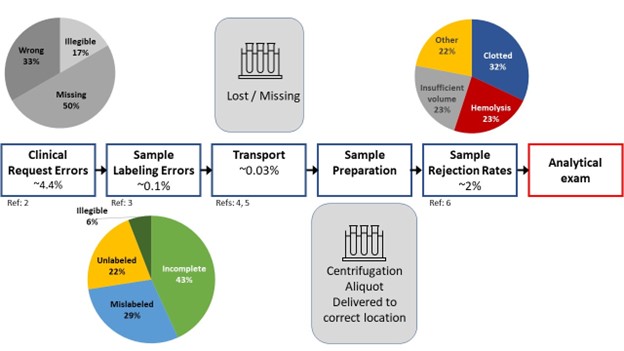
The challenges that face the laboratory include identifying potential errors that occur outside of their four walls that are unseen and may result from practices they have little control over. Electronic order entry systems have revolutionized laboratory requisitions diminishing request errors and streamlining specimen accessioning processes (2). Labeling errors diminish when computer generated labels utilizing barcode technology are utilized compared to relying on manual handwriting methods which have been reported as high as 18-26% (3, 7).
Laboratories have gotten creative by using various mechanisms to detect potential preanalytical problems for which they can investigate and request a redraw when needed. These solutions include providing decision support at electronic order entry to guide test utilization, automated analytical systems to identify hemolyzed and clotted specimens, and middleware rules to look for irregular patterns of patient results (i.e. including, but not limited to, delta checks) that can signify a problem. Some of these systems detect errors reliably, while the efficiency of others are not well documented and may depend on the specifics of the practice and patient population served.
What actions can be taken to prevent pre-analytical errors? There are a few measures to consider including development of clear standard operating procedures (SOP), a rigorous training program, and automating/standardizing the process as much as possible so that doing the right thing reproducibly is easy and becomes second nature. In addition, developing quality indicators that can be used as an objective means to monitor consistency across settings and over time will help signal a problem. Ideally, all steps of the pre-analytical phase would be monitored including appropriateness of test requests, completeness of order requisition and information needed to perform testing, mis-IDs, specimens lost or damaged in transport, specimens rejection rates and causes, and improperly labeled or stored specimens. It’s important to set appropriate limits and take action when they aren’t being met.
An event reporting system that identifies areas for improvement is an important addition to the laboratory’s arsenal for error detection. Most laboratories are required to have one and most institutions utilize them as well. Complaints originate from both inside and outside of the clinical pathology department (both are important to investigate) and can identify common themes, allow determination of root cause, and corrective measures can be taken whenever possible. The root cause may require collaboration with non-laboratory stakeholders to address and prevent future occurrences. For all cases, it is important to close the loop with whomever submits the complaint. Acknowledging a problem and communicating the follow-up goes a long way toward improving customer satisfaction, even if the problem can’t be resolved or improved in a timely manner.
It is also important to acknowledge the difference between perception and reality when it comes to complaints and errors. Going through a formal root cause analysis can be informative and sometimes surprising. It is well worth the effort to collect data, even in a manual fashion if needed, to establish that a problem indeed exists and identify its cause; you may discover that the cause is what you least expect.
References
- Plebani M. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Biochem Rev 2012;33:85-8.
- Carraro P, Zago T, Plebani M. Exploring the initial steps of the testing process: frequency and nature of pre-preanalytic errors. Clin Chem 2012;58:638-42.
- Wagar EA, Stankovic AK, Raab S, et al. Specimen labeling errors: a Q-probes analysis of 147 clinical laboratories. Arch Pathol Lab Med 2008;132:1617-22.
- Dale JC, Novis DA. Outpatient phlebotomy success and reasons for specimen rejection. Arch Pathol Lab Med 2002;126:416-9.
- Goswami AP, Sankar Roy S, Goswami NN. Evaluation of Specimen Rejection rate in Hematology Laboratory. J Dent Med Sci 2014;13:1-4.
- Getawa S, Aynalem M, Melku M, et al. Blood specimen rejection rate in clinical laboratory: A systematic review and meta-analysis. Pract Lab Med 2023;33:e00303.
- Bhat V, Tiwari M, Chavan P, et al. Analysis of laboratory sample rejections in the pre-analytical stage at an oncology center. Clin Chim Acta 2012;413:1203-6.