Summary
DOI: 10.1373/clinchem.2009.134940
A 24-year-old woman with advanced Hodgkin disease received the standard dosing protocol for busulfan/cyclophosphamide before allogeneic hematopoietic stem cell transplantation (HSCT)1 from a matched unrelated donor. The target area under the plasma concentration vs time curve (AUC) for busulfan was set at 950 μmol · L−1 · min−1, near the low end of the therapeutic interval of 900–1350 μmol · L−1 · min−1. The patient’s body mass index (BMI) was 45.5 kg/m2 (height, 170.2 cm; weight, 132.0 kg), so the dose was based on the patient’s ideal body weight. The patient received 2-hour intravenous infusions of busulfan every 6 h for 4 days (16 doses total). The patient was concurrently prescribed multiple medications, including an immunosuppressant, an antiviral, an antifungal, an antidepressant, an anxiolytic, a β-blocker, and a muscle relaxant, as well as antibiotics, warfarin, opioids, and antiepileptic drugs.
Student Discussion
Student Discussion Document (pdf)
Kamisha L. Johnson-Davis,1 Gwendolyn A. McMillin,1* JoEtta M. Juenke,3 Clyde D. Ford,2 and Finn B. Petersen2
1Department of Pathology, University of Utah Health Sciences Center, Salt Lake City, UT; 2Intermountain Health Care, Salt Lake City, UT; 3ARUP Laboratories, Institute of Clinical and Experimental Pathology, Salt Lake City, UT.
* Address correspondence to this author at: ARUP Laboratories, 500 Chipeta Way, Salt Lake City, UT 84108. Fax 1-801584-5207; e-mail [email protected].
4 Nonstandard abbreviations: HSCT, hematopoietic stem cell transplantation; AUC, area under the plasma concentration vs time curve; BMI, body mass index; PK, pharmacokinetics; Css, steady-state concentration; SOS, sinusoidal obstruction syndrome; Vd, volume of distribution.
Case Description
A 24-year-old woman with advanced Hodgkin disease received the standard dosing protocol for busulfan/cyclophosphamide before autologous hematopoietic stem cell transplantation (HSCT)4 from a matched unrelated donor. The target area under the plasma concentration vs time curve (AUC) for busulfan was set at 950 µmol · L-1 · min-1, near the low end of the therapeutic interval of 900–1350 µmol · L-1 · min-1. The patient’s body mass index (BMI) was 45.5 kg/m2 (height, 170.2 cm; weight, 132.0 kg), so the dose was based on the patient’s ideal body weight. The patient received 2-hour intravenous infusions of busulfan every 6 h for 4 days (16 doses total). The patient was concurrently prescribed multiple medications, including an immunosuppressant, an antiviral, an antifungal, an antidepressant, an anxiolytic, a β-blocker, and a muscle relaxant, as well as antibiotics, warfarin, opioids, and antiepileptic drugs.
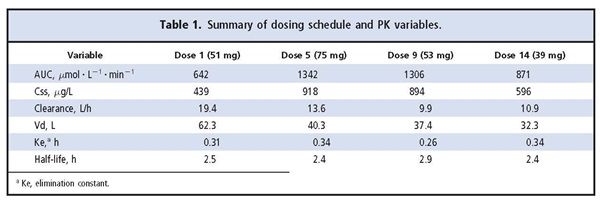
After the first dose (51 mg Busulfex®; Otsuka Pharmaceutical), timed plasma samples were collected after infusion to determine the AUC. Pharmacokinetics (PK) analysis was performed, and the AUC was determined to be 642 µmol · L-1 · min-1. According to these results, the predicted busulfan dosage required to achieve the target AUC was 75 mg per dose for the remaining 14 scheduled doses. Because of the large change in dosage, additional PK monitoring of busulfan was performed after the fifth dose (day 2). The fifth dose was selected to allow time to reach a steady-state concentration (Css) after the dosage adjustment and to avoid challenges in interpretation due to possible circadian variation in busulfan concentrations (1). Samples for monitoring were collected at the same time of day as the first monitoring dose. The AUC after the fifth dose was 1342 µmol · L-1 · min-1.
On the basis of the PK data from the fifth dose, dose 7 was changed from 75 mg to 53 mg, close to the original calculated dose of 51 mg. Of note is that the patient was started on an oral antifungal agent (fluconazole, 400 mg/day) after dose 6. Additional monitoring of busulfan was performed immediately after the ninth dose because of another change in dose and potential reduced clearance through busulfan interaction with fluconazole. The AUC after dose 9 was 1306 µmol · L-1 · min-1. The busulfan dose was decreased to 39 mg for the remaining doses because the AUC was near the high end of the therapeutic range and because clearance was reduced between the fifth and ninth doses. Monitoring was performed after the 14th dose to verify the adjustment; an AUC of 871 µmol L-1 · min-1 was observed. No further dose adjustments were made. The busulfan half-life and elimination constant were relatively stable throughout the dosing regimen (Table 1).
Questions to Consider
- When is the appropriate time to measure plasma busalfan concentrations in a patient? During the distribution phase of the drug or when the patient has reached a Css?
- Should busalfan dose adjustments be based on firstdose PK?
- What factors can affect drung absorption, distribution, metabolism, and excretion in patients?
- Can the coadministration of multiple drugs during busulfan therapy affect the PK of busulfan?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the July 2010 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2009.134940
Copyright © 2010 American Association for Clinical Chemistry