
For decades, body fluid testing has been a routine practice within clinical laboratories both large and small, and in the past 10−15 years especially, major strides have been made in understanding the practicalities of testing these fluids. The first Clinical and Laboratory Standards Institute (CLSI) document on the analysis of body fluids in clinical chemistry was published in 2007 (3). It summarized the usefulness of measuring a wide variety of analytes in a host of body fluids, and it was recently revised to include a robust tutorial on analytical validation, as well (1−5). Following the initial release of that CLSI document, the College of American Pathologists created a specific body fluid checklist item (COM.40620) to guide laboratories more specifically in the analytical validation of alternate specimen types, and academic research was published in the peer reviewed literature depicting performance characteristics of body fluid validation studies (6).
Despite these advances, many hurdles remain for the clinical chemistry laboratory performing body fluid testing today, from streamlining ordering to implementing literature-derived decision limits. Here we discuss important considerations that labs should take into account when facing these challenges, as well as the most up-to-date approaches to tackling them.
Ordering Practicalities: How Do You Say Potato?
First, let’s be real. Many of us have endured electronic health record (EHR) or lab information system (LIS) upgrades lately that also ripped off the band-aid of manual (often paper-based) ordering systems for body fluids. And honestly, this shift away from manual ordering isn’t always a good thing. In the haste of optimization, we may miss the simplicity of such manual processes because it turns out that body fluids are collected in so many different settings that electronic systems just do not seem to be as flexible as the old pen-and-paper systems. These settings include scheduled and unscheduled procedures in the operating room, in radiology, in the emergency department, at the bedside, and in exam rooms. This heterogeneity confuses the EHR and LIS and frustrates the people who rely on them.
The laboratory has a handle on which tests are most useful to perform for each of the specific body fluid types and has likely validated those types that are most often received and deemed useful. These become the “routine orderable” body fluid tests. There is a delicate balance between listing the entire dictionary of human anatomy and offering enough descriptors to differentiate the body fluid sources adequately. The lab should assist with designing the ordering screens for tests that are offered and provide input for configuration of the dropdown lists and/or ordering buttons to match those analytes often ordered together. For example, total protein and lactate dehydrogenase (LDH) would have pleural fluid listed first. There should also be an option for anatomic locations (e.g., left and right). Oops, the list just doubled in length. What is more, this must be done in concert with multiple laboratory sections (e.g., microbiology, cytology, hematology) that do not often share workflows or sometimes even nomenclature paradigms. If you want to have a good debate, ask your colleagues to explain the difference between body fluid type versus source. The challenge is satisfying everyone’s needs and being able to do it in two clicks. Good luck!
The logical way to offer these tests is within procedure order sets such as lumbar puncture, thoracentesis, arthrocentesis, etc. The cerebrospinal fluid (CSF) tests can be quite numerous, and this list can get rather unwieldy. Further stratification, in consultation with neurology and informatics, can help to organize the list into frequent (perhaps with tests selected by default), common (tests listed but not default selected), and rare (specialty and other esoteric tests may or may not be listed depending on how click happy your clinicians are). There may be further opportunity to subdivide by indication such as “infectious disease” and further differentiate tests for viruses, fungi, etc. The challenge here is coming up with a strategy that suits all users and whose comprehensiveness meets their expectations, and then convincing a programmer that it is worth it. Again—good luck!
Even after the above efforts, these order sets may or may not be available everywhere ordering is performed; thus, old school search and find also needs to work. When in doubt, get your downtime form out. The bottom line is that the cleaner and more thoughtful the upfront ordering is, the more useful the label logic that prints specimen labels can be, and the more organized the results display ultimately will be.
Processing Practicalities: You Want Me to What?
The goal of specimen processing is to prepare the sample for analysis and do all that is necessary to reduce the risk for error. When it comes to processing body fluids, one really must wonder how different it can be from processing other “normal” specimens received in the laboratory. What seems simple, however, is actually a fairly involved manual process (Figure 1).
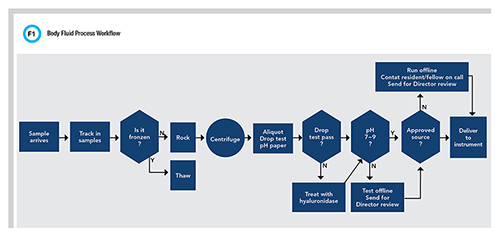
For example, we now appreciate that there is some amount of order and label reconciliation that may need to occur once a specimen arrives at the laboratory. Because these specimens are procured from a broad variety of settings and collectors, it is also reasonable to expect variations in container types and sizes.
It may be necessary for lab staff to aliquot the sample into instrument-ready tubes and affix a new label. Next, one may wonder if it is necessary to centrifuge the sample. At times, the cellularity of the sample may be minimal, and if the tube is destined for cell counting, of course, skip the centrifuge. However, body fluids often come with all manner of particulate, including cellularity, viscosity, and of course, color. A best practice is to centrifuge all body fluid samples and then dispense the supernatant into a new tube. Admittedly, this might be overkill, but at the very least the sample should not be left in contact with a noticeable pellet on the bottom of the tube that could interfere with instrument sampling.
This brings us to the next hurdle. Different instruments and sampling mechanisms can have different sensitivities to sample viscosity. Many manufacturers use mechanisms intended to sense fibrin clots that could potentially clog the probe or cause missampling. This triggers a warning to the operator not to trust these results, which ultimately prevents the reporting of inaccurate results. These mechanisms are calibrated for “approved fluids” such as serum or plasma as well as urine or CSF. However, body fluids can demonstrate extreme viscosity much higher than serum. The viscosity of water at 20°C is 1.0 centipoise (cP) and “healthy” serum is <1.5 cP. Serum from patients with hypergammaglobulinemia with or without symptoms of hyperviscosity syndrome will have a viscosity that is >3.0 cP and >10 cP in case reports. These highly viscous samples produce “clot” errors when aspirated on automated analyzers for testing. Laboratorians also describe fluids that are so viscous, they are unable to draw them into a normal pipette, much less an instrument set to aspirate 2 µL.
All this to say, when processing body fluids, it is wise to perform an assessment of the viscosity. The goal is to prevent potential damage to instrument probes and avoid the potential for mis-sampling that does not generate the error flag because of the wide difference between how the system was designed and what we are introducing from highly viscous body fluids. A quick and simple “drop test” using a pipette to evaluate the shape of the body fluid drop as it exits a pipette tip back into the test tube is sufficient (7). The rounder and less elongated the drop is, the closer the viscosity is to water and the less likely it is that the sample will cause issues with the instrument. What are the options for those viscous samples that do not pass the drop test? This has not been studied extensively; however, some suggestions are offered in the CLSI guideline. These include dilution, freeze-thaw, and treatment with hyaluronidase, which is an enzyme that cleaves the glycosidic bonds converting hyaluronic acid into less viscous monosaccharides (5).
When the laboratory is doing their manual viscosity check, it may also be prudent to consider a cursory check of pH, as this has been demonstrated to influence the measurable activity of enzymes (8). A low pH of <7 can be observed in many different sample types, some of which are obvious, such as gastric fluid, and some of which, like peritoneal fluid, aren’t. The concern is that low pH will diminish enzyme activity, rendering it unrecoverable with neutralization despite having elevated concentrations to start with. Therefore, result comments can be applied to help communicate the potential impact on results or cancellation of testing after clarifying the intent of testing with the provider. In summary, the hurdles for processing body fluids are the numerous manual tasks that do not fit into routine workflows. These can take significant time and effort, depending on the number of body fluid orders a testing laboratory receives.
Testing Practicalities: Mind the Gap
As mentioned earlier, there has been significant progress in understanding the practicalities of testing when it comes to body fluids. Guidance is now available for how to conduct an analytical validation (5). Numerous validation studies that demonstrate minimal matrix interferences and acceptable imprecision to meet clinical reporting needs have also been published.
The testing hurdle at this juncture is an underappreciation for limitations in the measurement range for assays that are designed for serum but used for body fluids. In many cases the measurement range is suitable for both. However, there could be a concern for analytes that have lower abundance in body fluids as compared to serum or plasma, such as LDH, total protein, albumin, and cholesterol. It is true that for a given instrument manufacturer, the laboratory can choose which application (serum versus urine versus other) best suits the intended measurement range. However, body fluid concentrations for analytes such as those listed above tend to be approximately 50% of serum concentrations, so the urine or CSF assay that measures orders of magnitude lower is typically not helpful.
In a recent body fluid method comparison between five different instrument vendors, Roche, Abbott, and Siemens produced measurable albumin results for all samples while Beckman and Ortho produced undetectable results at lower concentrations owing to the differences in measurement range (9). Table 1 summarizes the manufacturer’s measurement ranges and median albumin concentration in body fluids compared to serum.
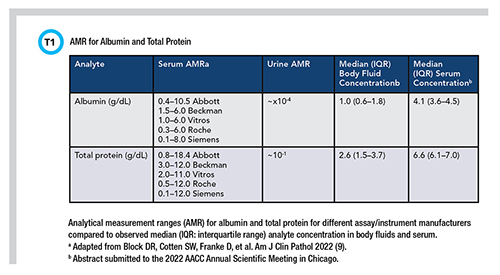
In summary, the hurdle today when testing body fluids is the appropriateness of the measurement range, which is reliant on instrument and assay manufacturers, and the willingness of the laboratory to pursue further modification of the assay to accommodate quantitation of body fluid analytes at a lower concentration so that meaningful interpretation can be made.
Reporting Practicalities: Did Someone Say Meaningful Interpretation?
As laboratorians, we strive to provide the most meaningful interpretive guidance for laboratory results that we possibly can. This is because we care deeply about satisfying patient needs and improving outcomes. This includes researching and verifying or establishing the most applicable reference interval data available to best serve each patient demographic we encounter. Reference intervals tend not to apply since most body fluids present in pathologic volumes amenable to collection are not associated with any state of “health.” Therefore, we rely on providing decision limits and interpretive comments to aid in the interpretation of body fluid test results. Table 2 summarizes three general approaches that have been taken to derive body fluid decision limits.
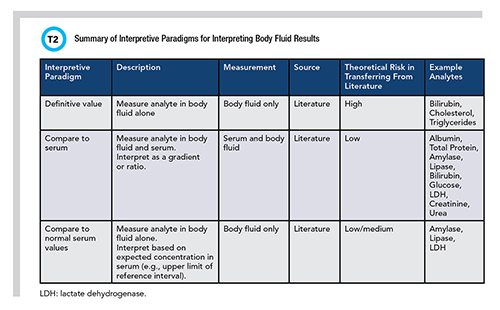
All of the decision limits identify presence or absence of a condition (such as triglycerides to identify chylous effusion) or differentiate one condition from another (such as transudate from exudate). Many decision limits were derived by comparing to the analyte concentration measured in serum. This opens a set of questions that are not easily answered, including how best to ensure a blood sample is collected and how near to the time of the body fluid collection that blood should be collected. Limited evidence suggests within 24 hours is best and for the reasons outlined above, ensuring this is done is nontrivial.
Many body fluid analytes have been studied, and decision limits are available in the published literature. The number of studies vary from the time-tested Light’s criteria to the scant number available for certain analytes like lipase (10). Most are rather dated, and their methods sections are often ill-described, which means that the largest risk in using published decision limits is transferring them into practice. On the other hand, when the concentration measured in body fluids is compared with a measured serum concentration or expected serum concentration, the risk is theoretically lower since one could postulate that the ratio between body fluid and serum should be equal, independent of method.
This assumption proved to be true for many analytes in a body fluid method comparison study performed by my lab; however, it did not hold true for every method and analyte studied (9). For example, creatinine demonstrated bias in several but not all body fluids, with one method at elevated concentrations (>3 mg/dL) that was not observed in serum or at lower concentrations, suggesting that the ratio of >1.0 to detect the presence of urine may need to be adjusted. A similar observation was made with glucose and urea suggesting further study is warranted.
Unfortunately, there is no gold standard body fluid method available so establishing a source of truth is impossible. Therefore, literature-derived decision limits are actually the best option for providing interpretive guidance for body fluid tests, but laboratories should be cognizant of the reputability of these decision limits and understand the risks in transferring them into practice today.
Conclusions
Body fluid test ordering is a challenge for everyone, so don’t feel bad if you experience problems with it. Body fluid processing is a time-consuming and manual endeavor that really is best considered a labor of love. Be mindful of the analytical sensitivity of the assays used to measure body fluid analytes by looking for an abundance of undetectable or “less than” results in body fluids not affecting the serum test. And lastly, laboratories should be wary of blindly transferring body fluid decision limits into practice from published studies, as bias between methods has been found that could affect interpretation.
Darci R. Block, PhD, DABCC, FADLM, codirects the central clinical laboratory and central processing at Mayo Clinic in Rochester, Minnesota. She also serves as the vice chair of informatics in the department of laboratory medicine and pathology. +Email: [email protected]
References
1. Light RW. The Light criteria: The beginning and why they are useful 40 years later. Clin Chest Medicine 2013;34:21−6.
2. Wians FH. To test or not to test? Opening Pandora’s box. Lab Med 2004;35:707.
3. Clinical and Laboratory Standards Institute (CLSI). Analysis of body fluids in clinical chemistry; Approved guideline. CLSI document C49−A. Wayne, PA: CLSI 2007.
4. Ritchie JC. Is it time to remove the stigma of LDT from body fluid chemistry assays? https://www.myadlm.org/science-and-research/scientific-shorts/2011/is-it-time-to-remove-the-stigma-of-ldt-from-body-fluid-chemistry-assays (Accessed July 26, 2011).
5. CLSI. Analysis of body fluids in clinical chemistry; Approved guideline. CLSI document C49-B. Wayne, PA: CLSI 2018.
6. Block DR, Ouverson LJ, Wittwer CA, et al. An approach to analytical validation and testing of body fluid assays for the automated clinical laboratory. Clin Biochem 2018;58:44−52.
7. Block DR, Florkowski CM. Body fluids. In: Rifai N, ed. Tietz textbook of clinical chemistry and molecular diagnostics. 6th Ed. St. Louis, MO: Elsevier 2018;925−.e35.
8. Nandakumar V, Dolan CT, Baumann NA, et al. Effect of pH on the quantification of common chemistry analytes in body fluid specimens using the Roche cobas analyzer for clinical diagnostic testing. Am J Clin Pathol 2021;156:722−7.
9. Block DR, Cotten SW, Franke D, et al. Comparison of five common analyzers in the measurement of chemistry analytes in an authentic cohort of body fluid specimens. Am J Clin Pathol 2022; doi: 10.1093/ajcp/aqab218.
10. Block DR, Algeciras-Schimnich A. Body fluid analysis: Clinical utility and applicability of published studies to guide interpretation of today's laboratory testing in serous fluids. Crit Rev Clin Lab Sci 2013;50:107−24.