Introduction
On January 1, 2016, the Centers for Medicare and Medicaid Services allowed the use of alternative QC testing based on risk management. Because the previous QC options were replaced by the new risk management process, laboratories that wanted to use alternative QC testing suddenly had the daunting task of validating and documenting their QC processes. CLSI and other organizations offered programs to help the laboratorians understand risk management and implement it in their QC programs. During follow-up sessions with laboratory scientists, many have acknowledged that working through the risk management process helped them to better understand and solidify their QC testing frequency.
Risk management can be used in other areas of quality management besides QC. Implementing risk management can help the laboratory:
- Ensure tests are working properly.
- Reduce the chance of errors.
- Improve efficiency.
- Save money.
- Ensure patients receive better care.
What is Risk Management?
Risk management is a process of identifying errors that can lead to patient harm. Figure 1 shows a sequence of events within a laboratory that could lead to a risk of harm for a patient: 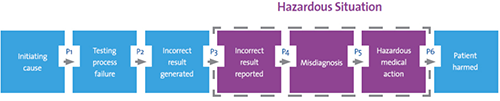
Figure 1. Sequence of Events That Could Lead to Patient Harm
Detecting or preventing the error at one of these steps can prevent patient harm from occurring. o Preventing an error from occurring in the first place is a more effective way of preventing harm than detecting the error because detection usually relies on human intervention, which is not always reliable.
Within the risk management process errors are identified, the risk of harm is estimated, actions are implemented to reduce the likelihood of the error (or detect it before it causes harm), and the effectiveness of the actions is monitored.The process of risk management attempts to answer four questions:
- What can go wrong?
- How bad could it be?
- How often could it happen?
- What can be done to mitigate or reduce the risk?
These questions can be asked of any laboratory process, not just in relation to QC testing frequency. For example, risk management principles can be used to reduce the cost of quality—one of the quality management system essential elements.
Applying Risk Management to the Cost of Quality
As the risk of patient harm increases, the cost of quality increases exponentially:
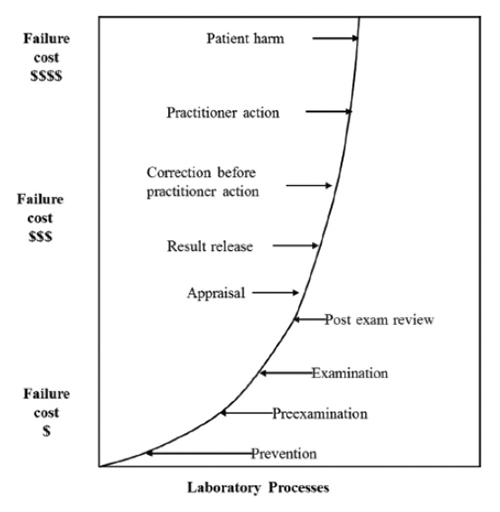
Figure 2. Failure Costs as a Function of Detection Point in Laboratory Processes. Berte LM. The cost of quality. In: Harmening DM, ed. Laboratory Management: Principles and Processes. 3rd ed. St. Petersburg, FL: D.H. Publishing & Consulting Inc., 2012:339-358; adapted for the laboratory from Wood DC, ed. Principles of Quality Costs: Financial Measures for Strategic Implementation of Quality Management. 4th ed. Milwaukee, WI: ASQ Quality Press; 2013. Reprinted with permission from D.H. Publishing & Consulting, Inc. Reprinted with permission from American Society for Quality, Quality Press © 2013 ASQ, www.asq.org. All right reserved. No further distribution allowed without permission.
According to CLSI’s QMS20, Understanding the Cost of Quality in the Laboratory,2 when erroneous examination results are not detected until just before result release, the laboratory has already incurred the costs of all the labor and materials in the preexamination and examination process for that sample, and upon repeating the testing, will incur the labor and material costs again. The highest and most adverse failure cost is when action taken as a result of a laboratory failure causes harm to the patient. From a patient health perspective and from a laboratory cost and liability perspective, any error that results in possible patient harm needs to be prevented.
Internal Failures and External Failures Need to be Considered
Internal failures are those errors that are caught and corrected inside the laboratory before the failure adversely affects patients. Internal failure costs are those incurred to fix a problem before delivery of the examination report. Some internal failure examples include sampling errors, insufficient or expired reagents or supplies, rework, retesting, reinspection, and instrument downtime.
External failures are problems detected outside the laboratory by physicians, nurses, patients, or other customers who receive faulty results, products, or services. These are the costliest because they include not only actual costs of producing the results, but also the costs of rectifying the problem, and sometimes considerable costs if the failure adversely affects a patient. Some examples of external failures include lost or erroneous test reports, customer complaints, misdiagnoses, and lawsuits.
The principles of risk management can be applied to any of these failures and measures can be put in place to reduce the risk of occurrence or the risk of possible harm. For example, instrument downtime, while an annoyance, needs to be properly planned and mitigated to prevent patient harm.
|
Failure
|
What can go wrong?
|
How bad could it be?
|
How often could it happen?
|
What can be done to mitigate or reduce the risk?
|
|
Instrument downtime
|
Physician does not receive necessary result, so patient does not receive necessary treatment.
|
Patient’s health worsens due to disease progression from lack of treatment.
|
The instrument has a history of being down 0.2% of the time. Given 5,000 patient results for this instrument per month, 10 patients could be harmed.
|
- Preventative maintenance could be performed more frequently to prevent downtime.
- A back-up instrument system could be placed into service when downtime occurs.
|
Laboratories with good quality management systems already track and record identified errors. This data is a good starting point for applying risk management. Measures can be put into place to reduce the risk of these known errors. Once the known errors are considered, the laboratory can then begin to tackle unknown errors. This is done by selecting a laboratory process and considering each step of the process sequentially. For each step, apply the four questions: What can go wrong? How bad could it be? How often could it happen? And what can be done to mitigate or reduce the risk?
By taking each laboratory process, step-by-step, and applying the four questions, laboratories can prevent and reduce errors and greatly improve their cost of quality.
References
- CLSI. Laboratory Quality Control Based on Risk Management; Approved Guideline. CLSI document EP23-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
- CLSI. Understanding the Cost of Quality in the Laboratory; A Report. CLSI document QMS20-R. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
Industry Insights articles are created and paid for by advertisers. The views expressed in these articles do not necessarily represent AACC’s views, and their inclusion in CLN is not an endorsement by CLN or AACC.