Introduction
Clinical analytical tests are carried out in laboratories, universities and research institutes worldwide, through analysis of human blood and other matrixes. In vitro diagnostic (IVD) tests are typically carried out in hospitals and clinics to investigate wide-ranging diseases and conditions and to monitor ongoing treatments. For many years, clinical scientists and clinicians have used immunoassays such as ELISAs (enzyme-linked immunosorbent assays) for analytical testing.
Concerns with Immunoassay Performance
In the past few years there has been growing concern about the accuracy of immunoassays including those for diagnostics. Immunoassays can be susceptible to interferences, usually manifesting as a lack of specificity, particularly for analytes with similar chemical structures. This is due to cross reactivity of the antibody with a similar species, which can cause inaccurate analysis, especially for low abundant analytes. This and related issues can lead to both false negative and false positive results, which can affect patient care as well as increasing costs and labor burdens for health authorities. Immunoassays can also show significant bias in results, between labs using the same assays as well as between assays sourced from different manufacturers.
The nature of the immunoassay technique also gives rise to another inherent issue with this approach. Antibodies are not available for every possible analyte that is measured or will be measured in the diagnostic lab and developing a new immunoassay can take months. So if a novel biomarker needs to be investigated, the immunoassay model is slow to react to such changing analytical needs.
Adoption of Alternatives
These concerns have driven the development of alternative testing methods based on mass spectrometry, which offers higher accuracy over immunoassays. Liquid chromatography with tandem mass spectrometry (LC-MS/MS) is particularly powerful for high-performance separation, identification and quantification of the small molecules, proteins and peptides commonly analyzed in a clinical diagnostic laboratory. These benefits for diagnostics are clear, but clinical laboratories have been slow to adopt LC-MS/MS. Part of the reluctance to switch technologies may be attributed to a traditional perception that only mass spectrometry experts could operate the instruments and analyze results. Accordingly, instrument providers have made many developments to improve the simplicity, ease-of-use and robustness of LC-MS/MS systems in recent years. These include the registration of the MS analyzers as general medical devices in many countries around the world, ensuring confidence that MS systems and consumables are developed specifically to be safe and effective for routine clinical diagnostic laboratories, and designed to bring down costs by delivering accurate, rapid and reliable results.
Benefits of Mass Spectrometry
The benefits of an LC-MS/MS approach versus a traditional immunoassay approach are numerous.
- Increased specificity – LC-MS/MS methodologies are based around the molecular structure and chemical fragmentation pathway of a molecule. Therefore, the specificity of a mass spectrometry approach compared to immunoassay is greatly enhanced
- Increased sensitivity – Sensitivity and specificity go hand in hand. Immunoassays can struggle with analytes in the picomolar concentration ranges, and for these “needle in a haystack” applications, their specificity can be poor. Mass Spectrometry is known and respected for its sensitivity in quantitation, and a powerful LC-MS/MS tool, such as the SCIEX Citrine MS/MS System can detect and quantify analytes at ultra-low concentrations with the specificity mass spectrometry provides
- Increased selectivity – One of the common misconceptions around mass spectrometry is concerns with its susceptibility to matrix effects. Sample preparation is often needed, but the selectivity of the LC-MS/MS approach confines that to a simple and automatable process. Powerful chromatography separates matrix from the analyte, and specific MS parameters detect and quantify the molecules of interest with confidence. What’s more, advanced mass spectrometry methodologies such as those offered by the SCIEX Citrine QTRAP MS/MS System allow ultimate selectivity from challenging matrices such as hair.
- Analyte Multiplexing – Advances in LC-MS/MS technology allow for the simultaneous detection and quantification of hundreds of analytes in a single injection. Moreover, improvements in LC system technology means that switching between methods using different LC chemistries is seamless and automatic.
- A powerful method development tool – LC-MS/MS is an ideal platform for the concept of the Lab Developed Test (LDT). This brings the full power of mass spectrometry into the hands of the routine diagnostic lab with the ability for the user to develop and deploy their own methods quickly and easily, reacting to changes in analytical demands in a timely fashion.
Ever improving ease of use – Manufacturers have been quick to perceive that LC-MS/MS is a technique requiring a high level of expertise. Extensive development in software and user interfaces, such as the SCIEX Cliquid MD platform, has brought this powerful analytical tool to the hands of users with minimal mass spectrometry experience. With that in mind, manufacturers understand that aftercare is paramount to the success of the laboratory, and customizable dedicated clinical training programs such as SCIEX University and support packages from SCIEX Now, peace of mind and confidence are ensured.

Figure 1. The Citrine LC-MS/MS mass spectrometer from SCIEX: a Medical Device
The Citrine LC-MS/MS system from SCIEX is the perfect example as a platform for immunoassay replacement. It offers ESI and APCI ionization options, an extended mass range up to m/z 2000, and a large linear dynamic range, making this the perfect tool for the measurement of a large variety of polar and non-polar biomarkers and metabolites in biological fluids, over a large range of concentrations. Also available with the SCIEX Triple Quadrupole Linear Ion Trap (QTRAP) technology, Citrine becomes a hybrid triple quadrupole/linear ion trap mass spectrometer - a unique, flexible MS/MS system that can accommodate a wide variety of both quantitative and qualitative LC-MS/MS workflows. It is the ability to use both triple quadrupole and linear ion trap scan functions on a single platform – and even within a single LC-MS/MS run – that makes the QTRAP system adaptable to a wide variety of both screening and quantitative tests. On the quantitation side, in some cases isobaric interferences cannot be differentiated by MRM alone, since the interferences may have the same exact mass as the target compound. In these cases, the ability to use second-order fragmentation (MS/MS/MS, or MRM3) provides highly specific measurements and can remove chromatographic interferences caused by isomers and background ions, without the need for extended chromatography and reduced throughput.
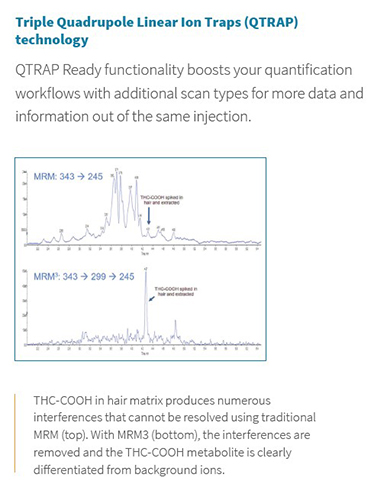
Figure 2. QTRAP technology enabling second-order fragmentation for THC-COOH in hair matrix
Immunoassays are still the mainstay of the clinical diagnostics lab but as can be seen, the benefits of a mass spectrometry approach are clear. Mass Spectrometry answers a significant number of the questions over immunoassays, and its reputation as an accurate and robust routine tool, while still maintaining powerful method development capabilities, is strong and growing. The future of mass spectrometry in clinical diagnostics and clinical research is assured.
Industry Insights articles are created and paid for by advertisers. The views expressed in these articles do not necessarily represent AACC’s views, and their inclusion in CLN is not an endorsement by CLN or AACC.