Advanced techniques encourage researchers to dig deeper and try to solve many questions related to their research. However, it is not always the case that we need to use sophisticated techniques to get more answers!
Our research focuses on prostate cancer and steroid metabolism. Prostate cancer is the most commonly diagnosed cancer and the second most common cause of cancer-related death among American men. Prostate cancer progression depends on continued androgen receptor (AR) activity. AR activation depends on two steroidal hormones, testosterone and dihydrotestosterone (DHT), which act as fuel for malignant cell growth; DHT is the primary androgen binding the AR in the nucleus. Androgen deprivation therapy (ADT), either by medical or surgical castration, is the choice for treatment. However, in most advanced cases, although the cancer initially responds to ADT, after time, it becomes resistant, and castration-resistant prostate cancer (CRPC) develops.
Recently, we studied the metabolism of abiraterone (Abi), a steroidal compound that is used to treat metastatic CRPC. It is given as the prodrug, abiraterone acetate. Abi blocks 17α-hydroxylase/17,20-lyase (CYP17A1), an enzyme required for androgen synthesis.
We have recently shown that Abi is converted by the 3β-hydroxysteroid dehydrogenase (3βHSD) isoenzyme to its Δ4, 3-keto structure (Δ4-abiraterone; D4A). We studied this conversion both in vitro and in vivo1. The structure of D4A is similar to that of testosterone and androstenedione, which will enable further metabolism (Fig 1). The downstream metabolites of D4A are either formed by 5α-reductase or 5β-reductase enzymes:
- 5α- reduced metabolites are 3-keto-5α-Abi, 3α-OH-5α-Abi and 3β-OH-5α-Abi
- 5β-reduced metabolites are 3-keto-5β-Abi, 3α-OH-5β-Abi and 3β-OH-5β-Abi
In vivo, D4A is more potent than Abi in inhibiting tumor growth in a mouse xenograft model. On the other hand, 3-keto-5α-Abi promotes tumor growth and is an androgen receptor agonist2; thus, it was important to determine and accurately quantitate all Abi metabolites in CRPC patients.
We developed and validated an LC-MS/MS method to separate Abi and its 7 steroidal metabolites in human serum3 (Fig 2). These metabolites are isomers: the four hydroxy metabolites are diastereoisomers, and the two 3-keto metabolites are isomers. Moreover 3- keto-Abi and Abi have the same mass transition and thus they are difficult to separate and analyze unless one uses a chiral column. Chiral columns pose difficulties though because no chiral column is considered universal and selecting the column requires many trial and error experiments. To avoid these problems, we developed a method that allowed us to separate the metabolites using a conventional reverse-phase C18 column with a mobile phase consisting of methanol, acetonitrile, and formic acid. The composition of the mobile phase along with the C18 column was the key to successful separation. Serum samples from patients with CRPC undergoing treatment with Abi acetate were collected. In all patient samples, Abi and all 7 metabolites were detected and quantitated.
This project integrates advanced techniques in clinical chemistry with cancer research; use of an advanced mass spectrometer allowed us to determine and accurately quantitate Abi metabolites. This approach will provide new knowledge about available therapeutic agents used in CRPC by identifying their metabolites and examining the role these metabolites play in prostate cancer. Such knowledge will lead to an understanding of why resistance develops and thus should improve treatment options.
- Li Z, Bishop A, Alyamani M, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 523, 347–351 (2015)
- Li Z, Alyamani M, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen. Nature 533, 547–551(2016)
- Alyamani M, et al. Development and validation of a novel LC–MS/MS method for simultaneous determination of abiraterone and its seven steroidal metabolites in human serum: Innovation in separation of diastereoisomers without use of a chiral column. J. Steroid Biochem. Mol. Biol. (2016) In press
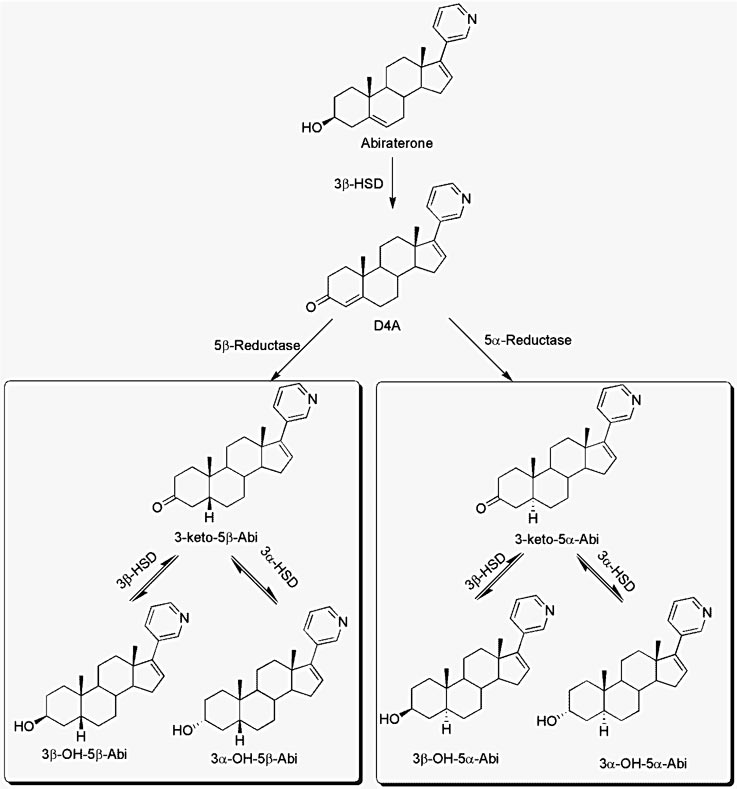 Fig 1 Steroidogenic enzyme metabolism of abiraterone 3αHSD: 3α-hydroxysteroid dehydrogenase
Fig 1 Steroidogenic enzyme metabolism of abiraterone 3αHSD: 3α-hydroxysteroid dehydrogenase
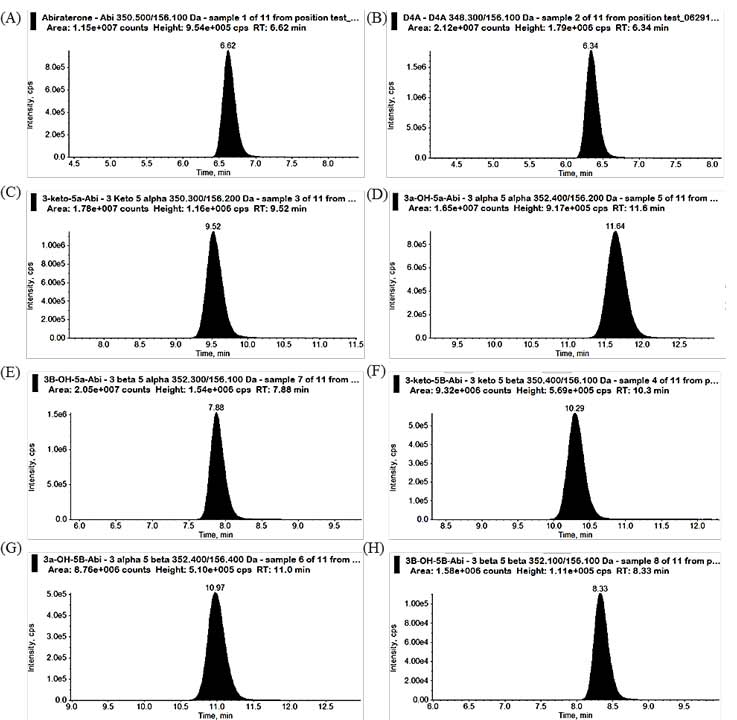 Fig 2. Chromatogram for abiraterone and its metabolites.
Fig 2. Chromatogram for abiraterone and its metabolites.