The field of clinical toxicology is overrun with illicit drugs, adulterants, unregulated supplements, and prescription medications that require screening and identification for medical management. A common method for untargeted drug screening on high-resolution mass spectrometers (HRMS) is information-dependent acquisition (IDA). In this method, only a user-defined number of the most abundant precursor ions per cycle (often 20) are selected for secondary fragmentation and library identification. This has come to show weaknesses in the clinical setting where compounds of interest may be in low abundance due to high potency, matrix effect, or polypharmacy background. We experienced this problem first hand on our HRMS drug screening assay when, occasionally, no fragmented MS/MS spectra would be collected for suspect compounds, effectively precluding confirmation. Understanding this limitation of IDA led us to seek alternative methods of unbiased data acquisition for drug screening.
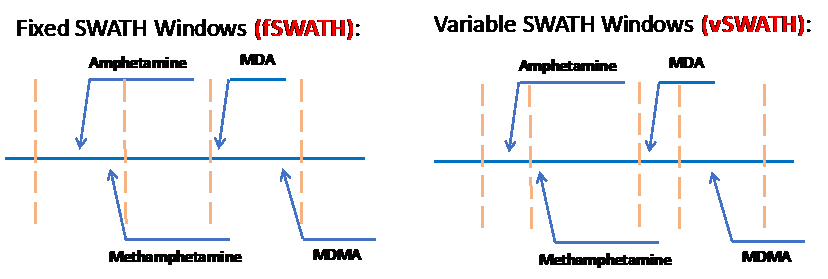
Sequential Window Acquisition of All Theoretical Fragment-Ion Spectra (SWATH) has emerged as a potential solution. SWATH data acquisition works by creating ‘windows’ where all precursor ions within a small Q1 mass range (e.g. 20 Da wide) are allowed to undergo fragmentation and identification so that no spectral data is lost (Fig. 1). This window then shifts to the next sequential range and repeats the process over and over until the desired mass range is covered for that cycle. One challenging factor of SWATH in the clinical setting is that drugs of a particular class will often have a similar chemical structure and mass. This means that similar drugs (e.g. amphetamine and methamphetamine) could fall into the same mass windows, confounding library searches. The solution to this problem lies in the fact that these SWATH windows are user defined -- meaning custom windows can be constructed to separate similar co-eluting compounds that would otherwise fragment together (Fig. 2). A variable SWATH window method (vSWATH) was adapted for clinical toxicology screening of our 169-compound drug library.
To assess if the unbiased data acquisition of vSWATH has benefits for analyzing clinical samples we put both methods head-to-head in limit of detection (LOD) studies in spiked urine (81 compounds) and assessed sensitivity and specificity in 50 previously-characterized patient urine samples. We found that both methods had similar LODs, with vSWATH being slightly more sensitive by 5-15 ng/mL in a majority of compounds.
When analyzing 50 clinical urine samples, we found that both untargeted methods confirmed 26 additional compounds not previously discovered by LC-MS/MS. The most interesting observation in this experiment was discovering compounds that were only found by one untargeted HRMS method, but not the other. Of the 7 missed by IDA (but found by vSWATH), 5 were due to a lack of MS/MS fragmentation, precluding library identification (as we previously experienced). In reviewing the IDA precursor ion scans for these compounds, we discovered that the drugs in question were in low abundance and therefore not selected for fragmentation and identification. Of the 5 compounds missed by vSWATH (but found by IDA), all were due to mass errors or no spectral library match.
Overall, our study concluded that vSWATH is at least equivocal to the current standard of IDA in the clinical setting, if not a bit more sensitive. It has the additional benefit of truly unbiased data acquisition so that all MS/MS spectra are acquired for identification of low abundance compounds or retrospective review. The limitation of SWATH is that it inherently has increased background noise or co-eluting compound spectra, which may affect library search and identification. Further studies will be useful in optimizing data-analysis criteria for vSWATH to increase identification confidence and expedite result review.
Figure 1
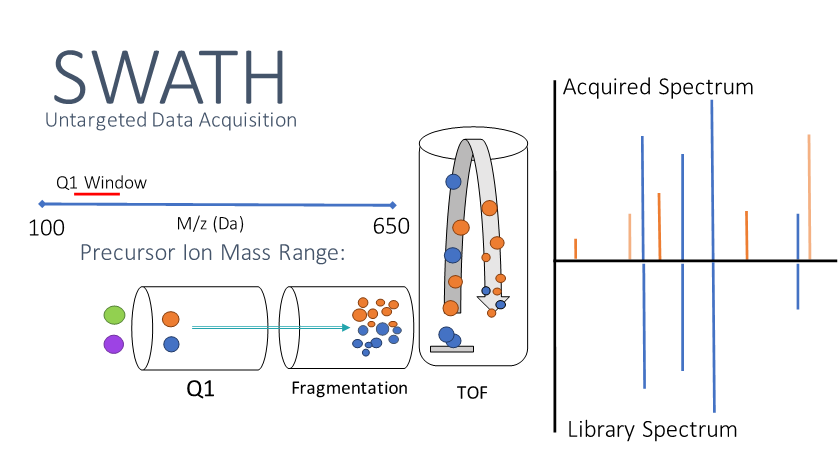
Figure 2
References:
- Wu AH, Colby J. High-resolution mass spectrometry for untargeted drug screening. Clinical Applications of Mass Spectrometry in Drug Analysis: Methods and Protocols. 2016:153-66.
- Thoren KL, Colby JM, Shugarts SB, Wu AH, Lynch KL. Comparison of Information-Dependent Acquisition on a Tandem Quadrupole TOF vs a Triple Quadrupole Linear Ion Trap Mass Spectrometer for Broad-Spectrum Drug Screening. Clinical chemistry. 2016 Jan 1;62(1):170-8.
- Colby JM, Thoren KL, Lynch KL. Optimization and Validation of High-Resolution Mass Spectrometry Data Analysis Parameters. Journal of Analytical Toxicology. 2017 Jan 1;41(1):01-5.