The Association for Diagnostics & Laboratory Medicine (ADLM) is concerned that the Food and Drug Administration (FDA) proposed rule to regulate laboratory developed tests (LDTs), in addition to current regulation under the Centers for Medicare and Medicaid Services (CMS), would hinder the ability of hospitals and academic centers to deliver timely diagnoses and care to patients.
To gauge the impact on patient care, ADLM recently conducted the first of several surveys that focused on hospital laboratories, with special attention to those that specialize in pediatric care. The responses underscored that FDA regulation of LDTs would impose new burdens that would directly affect care. In particular, many children’s hospitals stated they would be forced to outsource tests to commercial labs or switch to alternative FDA-approved tests, both of which could delay and impair diagnosis and treatment.
Of the 140 laboratories that responded to the survey, 87% stated they performed LDTs. Most have not, nor are they in the process of, developing a contingency plan to deal with FDA oversight. The reasons listed include the cost and administrative burden of FDA regulation, which would require them to cease performing LDTs, as well as the lack of familiarity with FDA rules compared to existing regulation under CMS.
Hospitals that have begun developing a contingency plan face a host of challenges:
- 69% of adult hospitals and 71% of children’s hospitals stated they would outsource many tests if LDTs become FDA regulated.
- More than 70% of children’s hospitals stated they would seek an exemption from FDA oversight, which is not included under the current FDA proposal.
- More than half of adult and children’s hospitals stated they would switch to FDA-approved tests in some instances.
- 86% of children’s hospitals plan to seek FDA approval for certain LDTs.
These problems threaten to diminish the overall quality of care these hospitals provide. Outsourcing tests can delay diagnosis and treatment, which is particularly concerning for tests used to diagnose and monitor genetic disorders in infants, where every minute of delay can contribute to brain damage and the risk of severe disability. Additionally, some FDA-approved tests, such as immunoassay drug screens, are less accurate or effective at capturing relevant information than their LDT counterpart.
If LDTs become FDA-regulated, children’s hospitals may be forced to make exceedingly difficult tradeoffs at the expense of patient care: To discontinue certain tests while feeling compelled to seek FDA approval for others—knowing that the cost of agency approval will likely diminish, not improve, the overall quality of care they provide.
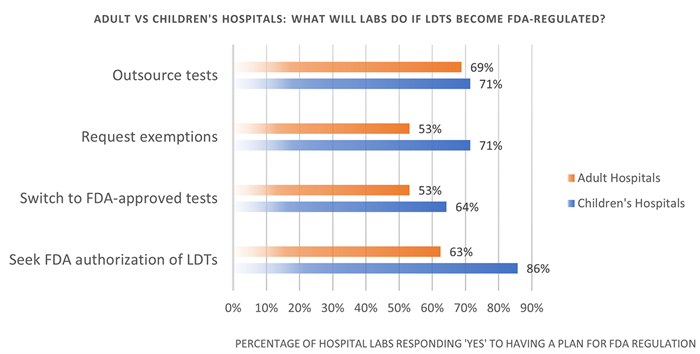
Figure 1: The actions adult and children’s hospitals with a contingency plan for FDA regulation stated they would take if LDTs become FDA-regulated.
View the full survey results
ADLM has also sent a letter about the survey results to Senator Bill Cassidy (R-La.), the Ranking Member of the U.S. Senate Health, Education, Labor, and Pensions (HELP) Committee, who recently put out a request for information on the regulation of clinical tests. View the letter here.
ADLM will continue to update this page with the latest survey data.