
The spillover of zoonotic diseases continues to make headlines across the globe, from SARS-CoV-2 to mpox to yellow fever. On June 26, 2023, the Centers for Disease Control and Prevention (CDC) distributed a Health Alert Network notification about locally acquired malaria in Florida and Texas—the first cases in the U.S. in two decades. Although the risk of locally acquired malaria remains low, CDC warns that the Anopheles mosquito vectors are found throughout many regions of the country and are capable of transmitting malaria if they feed on a malaria-infected person. Likewise, CDC must plan and establish access for IV artesunate, a first-line treatment for severe malaria cases.
CASES WITHIN THE U.S.
On June 23, 2023, the Texas Department of State Health Services (DSHS) reported a case of locally acquired malaria in a Texas resident with a history of working outdoors but no history of travel outside the state or country. DSHS has been working with local health departments to follow up on the case and determine whether other people have been exposed. To date, no other locally acquired malaria cases have been identified in Texas.
As of July 19, 2023, there have been seven locally acquired cases of Plasmodium vivax (P. vivax) malaria in Florida. These seven cases in Florida and the one in Texas show no evidence to suggest that the cases in the two states are related. All patients were promptly treated at area hospitals and are recovering. The Florida Department of Health has issued a statewide mosquito-borne illness advisory. Both states are advising everyone to take precautions to avoid mosquito bites.
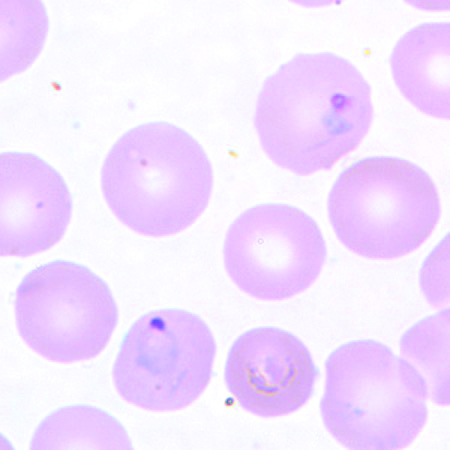
P. vivax in a thin blood smear.
LABORATORY DETECTION OF MALARIA
Diagnosed cases of malaria in the U.S. usually are from travelers returning from malaria-endemic countries. Due to its rare occurrence in the U.S., clinicians may be unfamiliar with the disease, thereby leading to a delay in diagnostic testing and patient care. Likewise, clinical laboratorians and public-health laboratorians may lack experience with malaria and fail to detect parasites upon blood smear microscopic examination.
Diagnosis can be challenging, given that signs and symptoms mimic other diseases and include fever, chills, sweat, headaches, muscle pains, nausea, and vomiting. Thus, confirmatory laboratory testing is critical and necessary for rapid treatment of the patient and to prevent further spread of infection in the community.
The gold standard for detecting malaria continues to be spreading a drop of the patient’s blood as a “blood smear” on a microscope slide and staining it to give the parasites a distinctive appearance. The microscopic examination of a patient’s blood is the most rapid, multiplex screening—and potentially confirmatory test—of the different protozoan stages of malaria, such as ring forms, schizonts, trophozoites, and gametocytes.
Rapid diagnostic tests (RDTs) offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not available. These immunologic RDTs detect antigens derived from malaria parasites. Like a common pregnancy test, these kits use a dipstick or cassette format to provide results in 2–15 minutes. Importantly, RDTs should always be validated.
Labs also can detect malaria using PCR-based molecular tests with species-specific techniques using common commercially available kits. While these tests are very sensitive and specific, the need for rapid diagnostics is critical for prompt treatment.
Serology testing can detect antibodies against malaria parasites, using either indirect immunofluorescence or enzyme-linked immunosorbent assay. However, these techniques are most often utilized in epidemiological studies of past infections, since they do not detect current infections.
Moreover, there are ongoing developments in malaria diagnostics. Flow cytometry and the overall category of nucleic acid amplification tests in PCR, loop‐mediated isothermal amplification, and molecular‐based point-of-care testing (POCT) is rapidly advancing in the world of all infectious-disease detection. Importantly, glucose-6-phosphate dehydrogenase (G6PD) testing is critical in G6PD-deficient phenotypes common in malaria-endemic areas. This is because the use of primaquine, an 8-aminoquinoline for the radical cure of P. vivax, is a major risk factor for hemolysis in these groups. POCT G6PD testing solutions have been commercialized in recent years and offer the potential to maximize the benefits of P. vivax radical cure while minimizing the risk.
In addition to ordering the most common diagnostic tests listed above, physicians should conduct an initial workup and request a complete blood count and a routine chemistry panel. These additional tests will be useful in determining whether the patient has uncomplicated or severe manifestations of the malaria infection, including severe anemia, hypoglycemia, renal failure, hyperbilirubinemia, and acid-base disturbances.
The risk of locally acquired, as well as, imported cases of malaria and other infectious diseases continues to increase due to climate change: Higher temperatures, heat waves, rainfall, and floods are all factors that create favorable conditions for mosquito populations. Enhanced surveillance for mosquito-borne infections and sustainable methods for controlling mosquito populations are critical public- health strategies that should be prioritized given the ongoing risk of locally acquired cases.
Rodney E. Rohde, PhD, MS, SM(ASCP)CM, SVCM, MBCM, FACSc, is a regents’ professor, university distinguished professor, honorary professor of international studies, global fellow, and chair in the CLS program at the College of Health Professions; associate director, Translational Health Research Center at Texas State University; and associate adjunct professor of biology at Austin Community College. +Email: [email protected]
Priya Dhagat, MS, MLS(ACSP)CM, CIC, CHEP, is an infection preventionist and the associate director of the System-wide Special Pathogens Program at New York City Health + Hospitals. +Email: [email protected]