
Many people become more active as they emerge into the warmth of spring and summer after enduring a long winter—and so do the ticks that often find them. Tick-borne diseases (TBDs) cause significant morbidity globally, including in the United States, where more than 50,000 cases are reported annually to the Centers for Disease Control and Prevention (CDC).
And that is just the tip of the iceberg when it comes to estimating the true burden of disease, since many cases go undiagnosed or unreported. With Lyme disease, for example, approximately 35,000 cases are reported to the CDC each year—but the true incidence of infection may be nearly tenfold higher, studies suggest (1). This underscores why it’s important for clinicians to hold appropriate suspicion for tick-borne infections, as informed by a patient’s geographic exposure history, time of year, and clinical presentation, and to apply relevant diagnostic testing and treatment. Taking these measures is essential not only for optimal patient care, but for the epidemiologic surveillance of these menacing vectors and the pathogens they spread.
This article summarizes the current state of diagnostic testing for Lyme disease and highlights recent progress toward fully automated testing, which will allow clinicians to make timelier, more accurate decisions.
TICKS AS DISEASE VECTORS
Numerous tick genera are capable of spreading bacterial, parasitic and/or viral pathogens to humans. Among these, Ixodes species are the most concerning, given their ability to transmit seven human pathogens, including Borrelia burgdorferi (the most common causative agent of Lyme disease in North America), Anaplasma phagocytophilum, Babesia species, Ehrlichia muris euaclairensis, and Powassan virus, among others.
Because Ixodes ticks can harbor all these pathogens, they carry a risk of cotransmission and coinfections. Among patients with Lyme disease, for instance, anywhere from 2% to 20% (depending on geographic location) are coinfected with Babesia microti, and up to 10% will also be positive for A. phagocytophilum (2, 3). Thus, testing for coinfections is warranted for many patients, particularly those at risk for babesiosis, since the treatment for this protozoan infection differs from that used for tick-borne bacterial pathogens.
DIAGNOSTIC TESTING FOR LYME DISEASE
While molecular methods remain the testing modality of choice for diagnosing most TBDs, this is not the case for Lyme disease. Molecular testing for detecting B. burgdorferi remains insensitive, largely due to the limited and transient bacteremia associated with infection. According to a recent metaanalysis, molecular testing of blood and cerebrospinal fluid for Lyme disease was associated with a median sensitivity of 18% and 22% across studies, with the highest sensitivity observed in synovial fluid (median, 77%) and erythema migrans (EM) tissue biopsies (median, 68%) (4).
Notably, EM rashes are considered pathognomonic for Lyme disease, and patients who present with such lesions (alongside appropriate geographic tick exposure) can be diagnosed clinically. Serologic testing is not indicated for these individuals, because the humoral immune response is still developing and would likely be undetectable by current assays. Molecular testing of EM biopsies is primarily beneficial in situations where the lesion does not have a classic “bull’s-eye” appearance or if there is a need to rule out rare conditions that mimic EM such as Southern Tick Associated Rash Illness (STARI).
Due to the limitations associated with molecular assays, diagnostic testing for Lyme disease remains based on detecting an anti-B. burgdorferi humoral immune response using an algorithmic, two-tiered testing approach. To appropriately utilize the assay and interpret the results, it’s important to have a clear understanding of immune response kinetics, alongside individual assay-specific caveats (e.g., B. burgdorferi antigens used, analytical specificity, etc.). Antibodies to B. burgdorferi can be detected 1−2 weeks after infection and typically peak over the first 1−3 months. Some individuals, particularly those with disseminated infections, will remain IgG seropositive for 6 months or longer.
Importantly, antibacterial treatment can affect the clinical sensitivity of serologic assays. This is particularly apparent in patients with EM, for whom prompt initiation of treatment can dramatically blunt immune response, resulting in up to half of these individuals remaining seronegative on convalescent testing (5).
Currently, there are two two-tiered testing algorithms endorsed by the CDC for diagnosing Lyme disease: the Standard Two-Tiered Testing Algorithm (STTTA) and the Modified Two-Tiered Testing Algorithm (MTTTA). Many assays have been cleared by the Food and Drug Administration (FDA) for use in either algorithm or both of them.
The Standard Two-Tiered Testing Algorithm
The STTTA was developed and widely implemented following guidance from the Second National Conference on Serologic Diagnosis of Lyme disease in 1994, which recommended initial testing of at-risk patients using an enzyme immunoassay (EIA) or immunofluorescence assay (IFA). For seronegative patients, clinicians were encouraged to either consider an alternative diagnosis or, in the instance of recent exposure (≤ 30 days), to repeat testing on a convalescent sample and assess for seroconversion. For initially seropositive patients, the guidance recommended reflexive testing by separate, anti-B. burgdorferi IgM and IgG immunoblots, with IgM testing indicated only for patients with less than 30 days of symptoms.
The IgM and IgG blots have unique antigen combinations and interpretive criteria that have often led to confusion among both clinicians and patients. IgM immunoblots are considered positive if they detect antibodies against at least 2 out of 3 possible B. burgdorferi antigens (23 kDa, 39 kDa, 41 kDa), whereas IgG blots are considered positive if they detect at least 5 out of a possible 10 antigens (18 kDa, 21 kDa, 28 kDa, 30 kDa, 39 kDa, 41 kDA, 45 kDa, 58 kDa, 66 kDa, 93 kDa).
Importantly, these criteria are only applicable for North American immunoblots, which are based on antigens specifically from B. burgdorferi strain B31; these blots and criteria are not appropriate for, and would not detect immune responses against, other Lyme-disease-causing Borrelia species.
The Modified Two-Tiered Testing Algorithm
In 2019, the CDC endorsed the MTTTA, which essentially replaces the standard algorithm second-tier IgM/IgG immunoblots with either a single, total antibody EIA or separate IgM and IgG EIAs. These second-tier EIAs should be based on B. burgdorferi antigens that differ from those used in the first-tier EIA. The MTTTA offers numerous advantages over the STTTA. Perhaps most importantly, it has improved sensitivity, particularly for patients with early Lyme disease.
Irrespective of the MTTTA assay combination, its sensitivity
is 10%−30% higher in patients with EM as compared to the STTTA (56%−74% vs. 41%−58%, respectively) (5). At later stages of infection, sensitivity is equivalent between the algorithms, approaching 100% in patients with late Lyme disease. Specificity is likewise similar.
Additionally, because the many first- and second-tier assays for the MTTTA are not specific to B. burgdorferi B31, they likely detect antibodies to a wider variety of Lyme-disease-causing Borrelia species, potentially eliminating the need for multiple serologic assays (6, 7). More studies are needed to better define the accuracy of MTTTA assays as a sensitive approach for pan-Borrelia antibody detection for Lyme disease.
The MTTTA also eliminates any confusion associated with immunoblot interpretation, since this approach provides only qualitative “positive,” “negative,” or “equivocal” results. Another key advantage is that the MTTTA can fully automate the entire algorithm—which would allow a larger number of laboratories to perform testing for all tiers onsite, rather than sending samples out to reference laboratories for second-tier immunoblot testing.
LYME SPECIALTY LABORATORIES
Any discussion of testing for Lyme disease must deal with assays offered through “Lyme specialty laboratories.” These facilities offer a variety of assays, including both novel, lab-developed tests and FDA-cleared assays to which the labs apply alternative interpretive criteria. Importantly, while the CDC’s recommended criteria for interpreting FDA-cleared tests is supported by extensive studies, the same is not true for many assays offered through Lyme specialty laboratories, which
often are not evaluated independently or assessed in the peer-reviewed literature.
One review that compiled performance data on some of these assays found that such testing is not well validated, and, concerningly, has limited sensitivity and specificity (8). Consequently, results from Lyme specialty laboratories are not recommended by the CDC and should be interpreted with caution. Whenever possible, they should be confirmed using well validated methods.
AUTOMATION OF LYME DISEASE TESTING
The expanding prevalence of Lyme disease has led to an increase in annual testing volumes, which has in turn driven demand for automated screening options. While modern clinical laboratories have many levels of automation available for Lyme disease screening, there are a limited number of assays offering fully automated, random-access instrumentation, which is the goal for many facilities handling high screening volumes, particularly amid ongoing staffing shortages (Table 1).
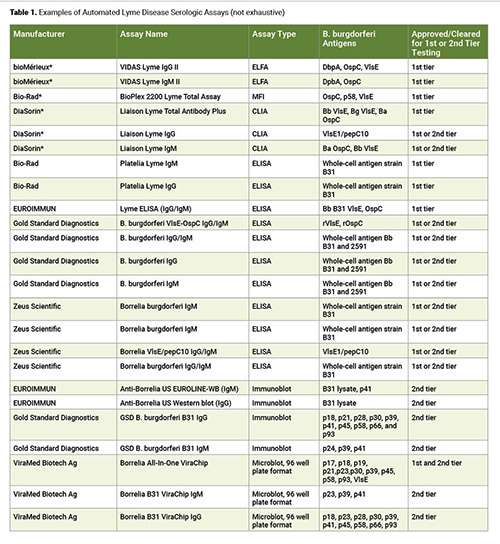
The available automated, random-access assays include those that differentiate IgG from IgM, as well as total immunoglobulin assays, which are often specialty instruments with testing menus focused on infectious disease serology and autoimmune diseases. This poses a challenge for smaller hospital laboratories in rural areas with a high prevalence of Lyme disease. These institutions may lack the financial resources to implement such platforms for the sole purpose of performing one or two assays.
The majority of available serology tests for Lyme disease are ELISA-based assays (examples in Table 1), which are amenable to batch testing automation through manufacturer or third-party ELISA automation processors. ELISA automation platforms offer labs some flexibility, since the menu of additional assays that can be placed on them is extensive and instrument costs are often more modest than random-access platforms.
The introduction of the MTTTA has enabled improvements to second-tier testing workflows that go beyond the processing enhancements made to immunoblot testing over the years. Initially, second-tier testing for the STTTA relied on classic Western blots that used whole-cell lysate. These not only required manual processing, but necessitated visual interpretation of the results for the presence or absence of bands by a technologist.
By contrast, contemporary immunoblots can use recombinant antigens that are mechanically applied, or “stamped,” onto nitrocellulose membranes, which are subsequently processed on semi- or fully automated instruments (examples in Table 1). Nevertheless, the process remains somewhat cumbersome, requiring specialized equipment to process the blots and limited assay menus to leverage introduction of this testing in-house.
Additionally, while many blotting methods can now be more objectively interpreted using optical densitometry scanning, some assays may still require a human to make the final visual adjudication for the presence or absence of bands, in accordance with CDC’s recommendations, especially when densitometry interpretations are inconclusive.
The MTTTA has not completely eliminated the need for batch testing of second tier assays, but it has significantly expanded the potential for automation and eliminated the need for visual interpretation. The same ELISA automation processor used in first-tier testing also can be used for second-tier testing, improving instrument utilization and turnaround time compared to send-out confirmation by immunoblotting. ELISA-based assays approved for MTTTA are still limited, and to date many are based on whole-cell sonicates. For now, only one random-access fully automated analyzer is currently approved for use for both tiers of the MTTTA.
It’s worth noting that, in the last year a Phase 3 clinical trial was initiated for the Pfizer and Valneva vaccine against B. burgdorferi. Known as VLA15, this vaccine is a multivalent, recombinant protein vaccine that targets the outer surface protein A (OspA) of six different B. burgdorferi serotypes. Although OspA is not present in many of the recombinant screening assays, potential cross-reactivity with assays using whole-cell sonicates is of concern.
IMPORTANCE OF TIMELY LYME DISEASE TESTING
Being able to accurately and promptly diagnose Lyme disease is increasingly important as prevalence rises. While Lyme disease is not itself life-threatening, it is common in differential diagnoses that may include more critical conditions—raising the stakes for making the correct diagnosis, particularly in summer months, when patients’ possible exposure to B. burgdorferi is high.
For example, in children presenting with a swollen, painful knee without a history of trauma to the site, the differential diagnosis includes septic arthritis—which often includes a trip to the operating room—in addition to Lyme arthritis and other conditions. Without rapid, readily available screening for Lyme disease, these pediatric patients may receive surgical intervention for an issue that could have been treated medically. In less critical cases, where the probability of Lyme disease is high, clinicians will often empirically treat for Lyme before testing results are available, leading to the potential overutilization of antibiotics.
Offering first-tier LD testing in-house is increasingly common in high prevalence areas, with second-tier testing sent out to reference laboratories. During the summer months, this process can take days to weeks, thereby forcing clinicians to make diagnostic decisions based on first-tier testing alone. This can contribute to over-treatment and missed alternative diagnoses.
MTTTA’s improved sensitivity in early disease and ability to perform batch automation on both tiers represents important progress towards providing clinicians the information they need as quickly as possible. However, we still lack choice in random-access, fully automated MTTTA assays, the primary benefits of which are rapid, same-day testing for both tiers.
Continued development of recombinant, random access, fully automated MTTTA assays will give clinicians access to more timely and accurate diagnostic information, resulting in better care for patients with Lyme disease and other conditions included in the differential diagnosis.
Sarah Wheeler, PhD, FADLM, is an associate professor in the department of clinical chemistry at the University of Pittsburgh Medical Center. +Email: [email protected]
Elitza S. Theel, PhD, is a professor in the department of laboratory medicine and pathology at the Mayo Clinic. +Email: [email protected]
REFERENCES
- Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014;59:676-81.
- Wormser GP, McKenna D, Scavarda C, et. al. Co-infections in persons with early Lyme disease, New York, USA. Emerg Infect Dis 2019;25:748-52.
- Horowitz HW, Aguero-Rosenfeld ME, Holmgren D, et al. Lyme disease and human granulocytic anaplasmosis coinfection: Impact of case definition on coinfection rates and illness severity. Clin Infect Dis 2012;56(1):93-9.
- Ruzic-Sabljic E, Cerar T. Expert Rev Mol Diagn 2017;17:19-30.
- Branda JA, Steere AC. Laboratory diagnosis of Lyme borreliosis. Clin Micro Rev 2021;34:e00018-19.
- Marques AR, Strle F, Wormser GP. Comparison of Lyme disease in the United States and Europe. Emerg Infect Dis 2021;27:2017-24.
- Branda JA, Strle F, et al. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis 2013; 57:3333-40.
- Moore A, Nelson C, Molins C, et al. Current Guidelines, Common Clinical Pitfalls, and Future Directions for Laboratory Diagnosis of Lyme Disease, United States. Emerg Infect Dis 2016 Jul; 22:1169–77.