Syphilis, a chronic bacterial infection caused by the spirochete Treponema pallidum, is a sexually transmitted infection (STI). However, syphilis also can be acquired through vertical (mother-to-child) transmission, and congenital syphilis continues to be a global cause of infant mortality. Syphilis has been stigmatized for hundreds of years, and its varying presentations and stages have historically often made this formidable contagion difficult to recognize and diagnose.
Syphilis has early and late stages. Early stages occur within the first year following infection and late stages at greater than 1 year from infection. Early stages include primary (chancre), which if untreated will progress to secondary (rash) syphilis. These two stages are the most contagious. Early latent syphilis is asymptomatic but acquired within the last year and is considered an early stage of syphilis. Late stages occur if syphilis remains untreated and include latent syphilis (asymptomatic) and the symptomatic tertiary syphilis.
Today standard clinical laboratory testing detects syphilis easily, and the infection when detected at an early stage is, generally speaking, easily treated with penicillin. Yet syphilis infections are rising. The U.S. experienced a more than four-fold increase in the incidence of primary and secondary syphilis cases between 2000 and 2017. This increase occurred disproportionately among sexual and racial minorities, including men who have sex with men (MSM) and Black men (1). Among MSM, approximately 50% of syphilis cases have HIV coinfection (2). 2016 data from Europe mirror these statistics (3).
The U.S. has seen increases in congenital syphilis cases in more recent years owing to increased incidence among females, a trend not observed in the European Union (1). Congenital syphilis is of particular concern as it leads to increased rates of miscarriage, stillbirth, and significant birth defects that might not be evident until early childhood or adulthood (4). The best treatment for congenital syphilis is prevention by detecting and treating the infection in women early in their disease course.
Only with thorough and effective screening can syphilis be treated and its spread mitigated. Clinical laboratories play a key role in reducing the burden of syphilis through early diagnosis, improved prenatal screening, and enhanced testing availability to all populations.
Laboratory Screening for Syphilis
Most laboratories offer serologic detection of syphilis. Unfortunately, the assays and their interpretation are complex and often misunderstood by physicians and patients. Because no one single test definitively diagnoses untreated syphilis, laboratories rely on testing algorithms. Importantly, the appropriateness of each test sequence can vary based on the laboratory and population.
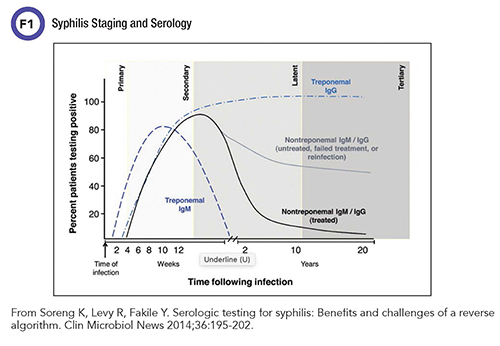
Serologic assays for syphilis diagnosis fall into two categories: non-treponemal and treponemal (Figure 1). Nontreponemal assays date to 1906, when August Paul von Wassermann in Germany described the first serologic test for syphilis based on complement fixation, later termed the Wassermann test or Wassermann reaction. This test was the basis for refinements that led to the Venereal Disease Research Laboratory (VDRL) test and the Rapid Plasma Reagin (RPR) test.
Nontreponemal assays assess antibodies against antigens released during the cellular damage caused by syphilis infection or released by the treponemes. VDRL assays utilize cardiolipin- and lecithin-coated cholesterol particles as antigens which, when bound with antibodies in the serum, are measured via microscopic agglutination. RPR assesses macroscopic agglutination of cardiolipin-coated particles following binding with serum antibodies. Efforts to standardize the VDRL and RPR assays have led to the use of synthetic antigens for antibody detection, which have replaced the cardiolipin and lecithin that were previously derived from animal tissues with varying purity.
As indirect markers of infection, an important caveat to nontreponemal assays is that other pathophysiological conditions unrelated to syphilis can induce the antibodies they detect. Laboratories refer to these nonsyphilis positive VDRL/RPR tests as biological false positives. Biological false positives are common in autoimmune diseases, chronic liver disease, following acute febrile illness, or during pregnancy.
Despite this nonspecificity, nontreponemal tests remain valuable in assessing active disease because they are semi-quantitative, with results reported as a titer of antibody. This generally is thought to reflect the amount of antibody present and may correlate with the activity of the infection. Titers of nontreponemal antibodies usually fall following successful penicillin treatment for syphilis infection. However, as many as 20% of cases might not see a full decline in titers to seroreversion. These patients, referred to as serofast, do not lose their nontreponemal antibody response even after presumably effective treatment.
Significantly, nontreponemal titers tend to wane over time even without treatment, making these assays of limited clinical utility in later disease stages. The ability to monitor treatment efficacy in earlier disease stages makes these nontreponemal tests of important utility in syphilis treatment regimes.
Treponemal assays, on the other hand, are more specific and detect antibodies against the antigens specifically associated with T. pallidum. Treponemal tests are qualitative and do not provide titer information. They are usually reactive earlier than nontreponemal assays and will often remain positive for the rest of a patient’s life, even after successful treatment.
The most common treponemal assays are T. pallidum enzyme and chemiluminescent immunoassays, which can be specific for immunoglobulin (Ig) G or detect both IgG and IgM. Three manual treponemal assays—T. pallidum particle agglutination assays (TPPA), microhemagglutination test for antibodies to T. pallidum (MHA-TP), and fluorescent treponemal antibody absorbed test (FTA-ABS)—have been in use for decades.
TPPA and automated treponemal assays have surpassed FTA-ABS and MHA-TP in sensitivity and specificity, leading to a decline in the use of FTA-ABS and MHA-TP (5). TPPA is still generally considered the most specific of the treponemal assays, though there is a paucity of information in newer automated assays compared to TPPA (5).
Conventional treponemal tests initially used whole organisms to generate the tests, causing nonspecificity thought to be related to antibodies against commensal microorganisms. Newer specific recombinant proteins and polypeptides have mostly replaced this approach, improving both the sensitivity and specificity of enzyme and chemiluminescent immunoassays.
Due to the unique characteristics of nontreponemal and treponemal assays, laboratories use both for diagnosis of active syphilis infection. A reactive nontreponemal assay might not be due to syphilis specifically, and a reactive treponemal assay might be indicative of past treated infection. The limitations of each assay pose added complexity to this diagnosis for some patients.
Use of the assays also has changed over time. Historically nontreponemal assays were inexpensive and quick, making them a standard first-line screen, followed by treponemal assays when the screen was positive. In more recent decades, the introduction of automated treponemal assays has provided a workflow and often a cost advantage compared to the manual process for nontreponemal assays. Now, the automation of nontreponemal tests (primarily RPR) has inspired debate around the most effective testing algorithm for syphilis screening.
Screening Algorithms
In the U.S., the Centers for Disease Control and Prevention (CDC) has two accepted algorithms: the traditional (or forward) and the reverse algorithm. The traditional algorithm calls for a nontreponemal assay to screen patients, with positive screens followed by a treponemal assay to confirm that the positivity is due to syphilis.
With automated treponemal assays came the reverse algorithm that screens patients with a treponemal assay. In this algorithm, positives are assessed for active disease by a nontreponemal assay; discrepancies between the two assays are resolved by an orthogonal treponemal assay in case of false positivity on the first treponemal screen.
The 2014 European guideline on the management of syphilis provides three possible algorithms. The first is the same as the CDC traditional (forward) algorithm. The second involves a variation on the CDC reverse algorithm, with a recommendation that reactivity in the first treponemal assay be confirmed with a second treponemal assay. The third is a combined initial testing of treponemal and nontreponemal assays.
Which algorithm is best? It depends. As a laboratory assesses possible algorithm implementation, the advantages and limitations of each algorithm are important to consider.
Forward testing, that is, starting with a nontreponemal test, has allowed laboratories to perform an inexpensive and comparatively rapid test before confirming positive samples with a more time-intensive and expensive treponemal test. Nontreponemal screening is unlikely to detect early, latent, or treated syphilis cases, as these often do not have nontreponemal reactivity. Laboratories using an IgG specific treponemal test might find that some patients are reactive by nontreponemal testing before treponemal testing due to the inclusion of IgM detection in the nontreponemal tests.
In laboratories and low-resource settings that lack automated testing, nontreponemal tests remain a mainstay of first line testing. Use of the forward algorithm also requires that labs perform only two tests, compared to the three tests needed for CDC's reverse algorithm.
The CDC and European Centre for Disease Prevention and Control (ECDC) reverse algorithms appear to have similar sensitivity, specificity, and accuracy (6). Despite the similarity in detection, the ECDC algorithm notably does not provide a distinction between active and treated or latent disease. This can be problematic for test reporting, as most countries report primary and secondary syphilis rates with laboratory testing being a key component of this determination (7).
There has been concern about increased false-positive results when using reverse-testing algorithms in low prevalence populations. However, with the advent of treponemal testing utilizing recombinant proteins and automated methodologies, the sensitivity and specificity appears to have improved and these assays are clinically useful for these testing algorithms (5).
Due to the large number of automated treponemal tests and the relatively short time that many have been on the market—as compared to the decades of experience with VDRL and RPR by a limited number of manufacturers—there is variability in the reports about increased false positivity in the reverse algorithm (5,6,8,9). In this article, the term false positives refers to patients never exposed to syphilis, but some papers in the literature consider past treated syphilis positivity in the screening algorithm to be a false positive as it requires no intervention.
The variability in the literature likely reflects population and assay manufacturer differences. This is one reason for there not yet being a clear answer on which algorithm is best. Laboratories already performing syphilis testing might find a retrospective data review informative in assessing algorithm changes or in implementing additional testing.
Apart from standard screening algorithms, laboratories serving hospitals with labor and delivery or pediatrics populations should consider congenital syphilis testing as well, as it presents unique testing challenges. For example, maternal treponemal and nontreponemal IgG antibodies will pass to the fetus. Serologic testing in the mother is immensely important, and early prenatal detection of syphilis and appropriate treatment is the best way to improve infant outcomes.
CDC recommends only nontreponemal tests for detection of congenital syphilis. Ideally a laboratory would draw a paired specimen from mother and baby and run the samples on the same assay to obtain nontreponemal titers. An infant with a nontreponemal titer fourfold higher than the mother’s is considered proven or highly likely to have congenital syphilis, though lack of this finding does not exclude congenital syphilis. CDC does not recommend cord blood testing for syphilis due to maternal blood contamination, and Wharton’s jelly within the umbilical cord is known to cause false-negative results.
Multiplex Testing: A Case Study
When we at the University of Pittsburgh Medical Center implemented a new multiplex test that allowed for simultaneous assaying of treponemal and nontreponemal antibodies, we assessed how physicians used this testing to determine if a reverse or dual-reporting algorithm would be best. In our chart review we found many questions and misinterpretations of syphilis testing in the reverse algorithm.
For example, patients with a positive treponemal IgG and TPPA and a negative RPR were referred for infectious diseases consults as syphilis positive despite a documented history of treated syphilis years earlier. Meanwhile, for patients with a negative treponemal IgG screen, clinicians subsequently ordered an RPR (inappropriately) that was positive in the setting of active autoimmune processes, requiring further testing and psychological stress. Adding to the confusion, syphilis and other STI testing had recently been converted from a test that required physician review and approval before being sent to the patient portal to an auto-sent test, available within hours of the laboratory resulting the testing.
After discussions with infectious diseases colleagues and assessing the current state of testing, we decided to proceed with dual reporting of total treponemal antibodies and RPR to be followed by batched TPPA when treponemal and nontreponemal results were discrepant. When we implemented dual reporting, we decided to provide interpretive comments to physicians, and by extension, to patients to lower the confusion that these tests can create. We created the interpretive comments in collaboration with the infectious diseases physicians we serve.
We then performed a retrospective review of the first 3 months of testing to determine the efficacy of our testing algorithm (n=4558) (10). We found a low prevalence, 0.4%, of active syphilis and a 1.1% prevalence of treated syphilis. We also chart-reviewed positive screens to help identify false-positive screens as well as early/latent disease.
The dual screening identified five cases of early/latent disease (0.1%) that the forward algorithm would have missed but the reverse or dual algorithms would have identified. We found a false positivity rate in total treponemal testing of 0.26%—0.1% in TPPA and 3% in RPR. Our population has a high number of prenatal screens and autoimmune diseases, both of which are more likely to have biological false positives in the RPR screen, a tendency we observed.
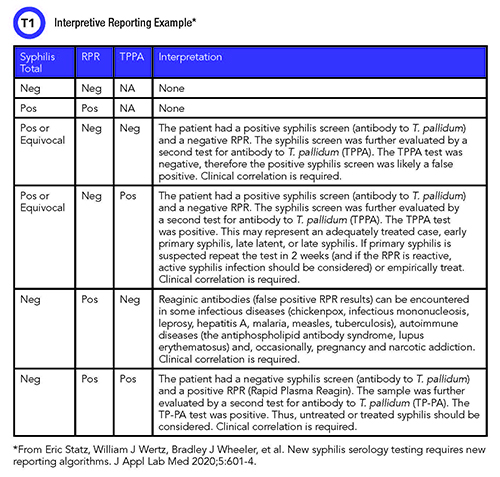
In our institution the dual screen has increased the number of TPPA confirmations we perform, but our physicians prefer having the information from both treponemal and nontreponemal testing. We maintained physicians’ ability to order RPR independent of the syphilis screen to allow for congenital syphilis testing as well as disease treatment management. The most important gain, however, has been in providing interpretive comments (Table 1). Both physicians and patients have benefited from the line or two of interpretation included to help clarify results.
Ideas for Clinical Laboratories
To determine the best algorithm, clinical laboratorians need to assess the technical capabilities of their laboratories, collaborate with infectious diseases colleagues, and review the populations they serve. Interpretive comments will significantly benefit both physicians and patients regardless of the algorithm implemented, particularly as many patients now receive their laboratory test results immediately via secure patient portals. Interpretive comments are also an opportunity to engage with infectious diseases colleagues to improve patient care and population health.
As syphilis rates continue to increase and enable the spread of HIV, it is vital that laboratories take a proactive role in improving syphilis screening to facilitate early detection and intervention.
Sarah Wheeler, PhD, FADLM is an assistant professor in the department of pathology at the University of Pittsburgh School of Medicine, associate medical director of clinical immunopathology at the University of Pittsburgh Medical Center, and medical director of the automated testing laboratory at UPMC Children’s Hospital of Pittsburgh and UPMC Mercy in Pittsburgh, Pennsylvania. +Email: [email protected]
References
- Centers for Disease Control and Prevention. 2017 sexually transmitted diseases surveillance. https://www.cdc.gov/std/stats17/default.htm (Accessed October 23, 2020).
- Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: An assessment of contributing factors. Infect Dis 2019;12:1178633719883282.
- European Centre for Disease Prevention and Control. Syphilis–Annual epidemiological report for 2016. https://www.ecdc.europa.eu/en/publications-data/syphilis-annual-epidemiological-report-2016 (Accessed November 2, 2020).
- Stafford IA, Sánchez PJ, Stoll BJ. Ending congenital syphilis.
JAMA 2019; doi: 10.1001/jama.2019.17031.
- Park IU, Tran A, Pereira L, et al. Sensitivity and specificity of treponemal-specific tests for the diagnosis of syphilis. Clin Infect
Dis 2020;71:S13-S20.
- Tong ML, Lin LR, Liu LL, et al. Analysis of 3 algorithms for syphilis serodiagnosis and implications for clinical management. Clin Infect Dis 2014;58:1116-24.
- Petty-Saphon N, Brady M, Cullen G, et al. A proposal for changes to the European Union syphilis surveillance case definition using evidence from evaluations in Ireland. Euro Surveill 2019;24:1900311.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: A comparison at a large academic medical center. Pract Lab Med 2017;8:52–9.
- Binnicker MJ. Which algorithm should be used to screen for syphilis? Curr Opin Infect Dis 2012;25:79–85.
- Statz E, Wertz WJ, Wheeler BJ, et al. New syphilis serology testing requires new reporting algorithms. J Appl Lab Med 2020;5:601-4.