A Diagnostic Conundrum
A 74-year-old women was referred to an endocrinologist to assess her for probable Cushing’s syndrome. Measurement of the patient’s morning serum cortisol and salivary cortisol (elevated), combined with typical signs and symptoms, helped the clinician confirm that she had Cushing’s syndrome. To further assess the source of the elevated cortisol, the physician ordered plasma adrenocorticotropic hormone (ACTH) testing. Repeated several times over the course of 3-months, using the Siemens Immulite ACTH assay, these results consistently fell within the assay’s reference range (10–18 pg/mL, reference range 6–50 pg/mL). Imaging studies previously identified an adrenal mass (incidentally); however, based on the ACTH findings, additional imaging was undertaken to look for an ACTH-dependent cause, including a pituitary tumor. This second round of imaging did not reveal a pituitary mass. Having previously cared for a handful of patients in which the ACTH results did not fit with the patients’ clinical picture, the endocrinologist was suspicious of the ACTH results and contacted the clinical lab to further examine this case. Based on the clinician’s suspicion, the lab sent out the ACTH testing to a different lab, which used the Roche Elecsys ACTH assay. The plasma ACTH was undetectable by this alternate method.
Which ACTH results were correct? Did the ACTH results indicate ACTH-dependent or ACTH-independent cortisol secretion?
ACTH and Disorders of the Hypothalamic-Pituitary-Adrenal (HPA) Axis
Plasma ACTH measurements have an essential role in diagnosing and managing patients with disorders of the HPA axis. These measurements help differentiate between primary adrenal insufficiency, characterized by an elevated concentration of ACTH, and secondary adrenal insufficiency, indicated by a low concentration of ACTH. Once a physician establishes a diagnosis of Cushing’s syndrome, ACTH concentrations are critical in determining the underlying pathology, discriminating with excellent sensitivity and specificity between ACTH-dependent (pituitary or ectopic) and ACTH- independent (adrenal) forms (Figure 1). While plasma ACTH assays are neither standardized nor harmonized, generally speaking, ACTH concentrations less than 5 pg/mL are considered consistent with ACTH-independent Cushing’s syndrome whereas values greater than 20 pg/mL are consistent with an ACTH-dependent cause (1-2). Concentrations that fall within the range of 5–20 pg/mL, or “gray zone”, are less definitive but are commonly associated with ACTH-dependent causes (1-2). Given the divergent diagnostic interpretations associated within this narrow range of plasma ACTH concentrations, measurement precision and accuracy are critical.
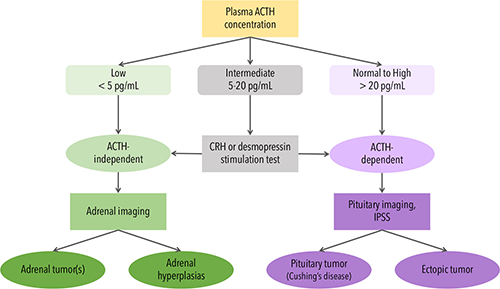
Figure 1: The Role of Plasma ACTH Measurement in Determining the Cause of Cushing’s Syndrome: CRH, corticotropin-releasing hormone; IPSS, inferior petrosal sinus sampling
Diagnostic Misadventures
In the case above, the endocrinologist, in consultation with the laboratory, relied on clinical acumen to adjudicate between the discrepant ACTH plasma results. With clinical findings pointing towards an ACTH-independent Cushing’s syndrome, only the Roche results were consistent with this clinical picture. The initial ACTH analysis performed on the Siemens system led to numerous repeated laboratory tests, unnecessary pituitary imaging, and a delayed diagnosis of an adrenal adenoma. Fortunately, based on the endocrinologist’s suspicion of an erroneous ACTH result and involvement of the clinical laboratory, unnecessary higher-risk procedures were avoided.
There are numerous other cases in the scientific literature detailing similar incidents where plasma ACTH was measured to clarify the etiology of disease and the result was discordant with the patient’s clinical presentation (3-7). In these cases, re-analysis of plasma ACTH on an alternate platform indicated that the initial ACTH result was likely inaccurate, eventually resulting in a revision of the diagnosis (3-7). Moreover, these cases highlight the impact on patients ranging from unnecessary additional routine lab tests to costly imaging and invasive testing such as inferior petrosal sinus sampling, and even to surgery (3-7).
As an example, in a case of a 21-year-old woman with consistently elevated plasma ACTH via the Siemens Immulite assay, the elevated ACTH finding led both physician and patient on a diagnostic misadventure that ultimately resulted in this young woman undergoing pituitary surgery (7). This surgery turned out to have been unnecessary; extensive investigations including ACTH analysis using alternate methods revealed that the patient had neither elevated plasma ACTH nor Cushing’s syndrome (7).
Approaches to Troubleshooting ACTH Immunoassay Results
After ruling out pre-analytical issues, the first steps in investigating cases of suspected spurious immunoassay results generally include repeat analysis, including repeat analysis after sample dilution and use of heterophile blocking reagents, and testing on an alternate platform. In a majority of cases in the literature, investigations of potential heterophile antibody interference in ACTH immunoassays rarely yielded informative findings, which begged the question: What exactly are the ACTH assays actually measuring?
In clinical ACTH sandwich immunoassays, the signal is proportional to the concentration of ACTH in the sample. For the sandwich, the assay’s capture and detection antibodies bind two epitopes (or the immunogen sequence if using polyclonal antibodies) on ACTH. An immunoassay’s design can create different susceptibilities and potential sources of interference, that is, 1-step v. 2-step, use of monoclonal and/or polyclonal antibodies, antibody sequence (species), and epitope locations on the analyte, among other considerations. ACTH assays are not harmonized, with no available reference material or reference method to assist in such an endeavor. While modest biases exist between different assays, the discrepancies noted in the published cases and outliers from our own method comparisons exceeded the expected bias between methods.
When a physician orders plasma ACTH, they expect that the result will reflect the concentration of biologically active intact ACTH (iACTH), a 39-residue peptide. ACTH is derived from precursor proteins, pro-opiomelanocortin (POMC) and pro-ACTH. ACTH can be further cleaved by endogenous proteases in tissues to form an N-terminal biologically active fragment, α-melanocyte-stimulating hormone (aMSH, comprising residues 1–13) and a C-terminal fragment termed corticotropin-like intermediate peptide (CLIP, residues 18–39). N-terminal residues 1–24 of ACTH are sufficient for biological activity and thus provocative testing to stimulate the release of cortisol by the adrenal glands uses synthetic polypeptides from this region (cosyntropin/Synacthen). These synthetic analogues, as well as endogenous ACTH fragments and precursors, can result in falsely elevated or falsely decreased ACTH results when measured by immunoassay, and the nature of the interference (positive or negative) can vary by assay for the same interferent (8).
Selective Measurement of Biologically Active, Intact ACTH
To overcome challenges associated with ACTH immunoassays, we developed a high performance liquid chromatograph mass spectrometry (HPLC-MS/MS) assay to quantify iACTH (8). Using HPLC-MS/MS enabled us to selectively detect and quantify various forms of ACTH, most critically the biologically active iACTH (Figure 2). This HPLC-MS/MS assay also enabled us to side-step interferences common to immunoassays including heterophile antibody interference and interference from closely related molecular isoforms—in this case ACTH precursors and fragments. After identifying several cases with discrepant ACTH results as measured on the two immunoassays most commonly used in North America (Roche Elecsys and Siemens Immulite)(8), we used the iACTH HPLC-MS/MS assay to help us answer the question: Which, if any, immunoassay result reflects the concentration of iACTH in a patient’s plasma sample? Consistent with the previously published literature correlating ACTH assay results with clinical findings, in our series of cases only the Roche assay results were highly positively correlated with the iACTH concentration determined by HPLC-MS/MS (8). In turn, these results indicate the Siemens Immulite was measuring something other than the intended measurand.
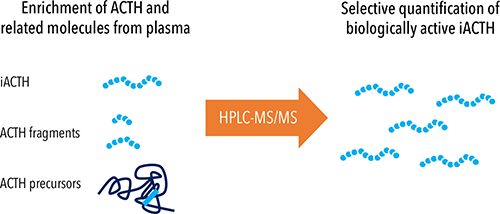
Figure 2: Selective Quantification of Biologically Active Intact ACTH by HPLC-MS/MS
Troubleshooting Advice and Future Prospects
When investigating potential causes for assay interferences, laboratorians typically start by repeating analysis using the same assay, followed by dilution studies and the use of heterophile block tubes. Testing on an alternate platform often involves sending out the samples and for this reason is usually one of the last steps. Based on the current available data and our experiences, we recommend that labs investigating an ACTH result prioritize troubleshooting via an alternate assay. This prioritization is driven in part by the limited volume often available for alternate/repeat testing; unlike the majority of analytes, ACTH requires special preanalytical conditions (collection into a chilled tube and frozen immediately prior to analysis) due to rapid proteolytic degradation. The literature supports this approach, indicating that for ACTH, testing for antibody-mediated interference is often uninformative. We also recommend making multiple aliquots (as is reasonable with the volume of plasma remaining), freezing them at -70 C and thawing an aliquot immediately before use when troubleshooting.
Although this is the simplest troubleshooting strategy currently accessible to most laboratories, we have found MS to be the only tool capable of providing empirical evidence of the true iACTH concentration when ACTH results and the clinical picture are discrepant. Development of the iACTH HPLC-MS/MS assay also highlighted the need for a commutable ACTH reference material to support harmonization of ACTH immunoassays and future standardization efforts. Currently, we use the iACTH HPLC-MS/MS assay in our ongoing investigations to determine the specific cause(s) of the observed interference in ACTH immunoassays, with the aim of facilitating analytical improvements in contemporary ACTH immunoassays.
Role of Clinical Chemists
One of the ongoing responsibilities for clinical chemists is being proactive in engaging our physician colleagues regarding any factors that could affect how they interpret test results. It can be difficult for a laboratorian to recognize a spurious ACTH result because doing so requires correlation with a patient’s complete clinical picture, information which might not be readily accessible. Similarly, physicians might have difficulty deviating from management plans informed by the ACTH result, based on perceived confidence in the lab result (7). As such, it is critical to increase awareness of the issue from the perspective of both the physician and clinical laboratorian. In our experiences, we have found success by engaging with these communities via grand rounds, case conferences, laboratory-focused didactics, and conference presentations.
Ultimately, laboratorians, physicians and assay manufacturer share the same goal: improving the quality of care for our patients.
Zahra Shajani-Yi, PhD, DABCC, FADLM, NRCC-CC, is technical director of Chemistry at LabCorp San Diego in San Diego, CA. This work took place while she was medical director of Esoteric Chemistry at Vanderbilt University Medical Center and an assistant professor at Vanderbilt University School of Medicine +Email: [email protected]
Mari L. DeMarco, PhD, DABCC, FADLM, FCACB, is a clinical chemist at Providence Health Care and a clinical associate professor at the University of British Columbia in Vancouver, Canada. +Email: [email protected]
References
1. Raff H, Carroll T. Cushing's syndrome: from physiological principles to diagnosis and clinical care. J Physiol. Feb 1 2015;593(3):493-506.
2. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing's syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol 2014;4(2):739-69.
3. Choy KW, Teng J, Wijeratne N, Tan CY, Doery JC. Immunoassay interference complicating management of Cushing's disease: the onus is on the clinician and the laboratory. Ann Clin Biochem 2017;54(1):183-184.
4. Donegan DM, Algeciras-Schimnich A, Hamidi O, et al. Corticotropin hormone assay interference: A case series. Clin Biochem 2019;63:143-147.
5. Grasko J, Willliams R, Beilin J, Glendenning P, Fermoyle S, Vasikaran S. A diagnostic conundrum: heterophilic antibody interference in an adrenocorticotropic hormone immunoassay not detectable using a proprietary heterophile blocking reagent. Ann Clin Biochem 2013;50(Pt 5):433-7.
6. Yener S, Demir L, Demirpence M, et al. Interference in ACTH immunoassay negatively impacts the management of subclinical hypercortisolism. Endocrine 2017;56(2):308-316.
7. Greene LW, Geer EB, Page-Wilson G, Findling JW, Raff H. Assay-Specific Spurious ACTH Results Lead to Misdiagnosis, Unnecessary Testing, and Surgical Misadventure—A Case Series. J Endocr Soc 2019;3(4):763-772.
8. Shi J, Dhaliwal P, Zi Zheng Y, et al. An intact ACTH LC-MS/MS assay as an arbiter of clinically discordant immunoassay results. Clin Chem 2019;65(11):1397-1404.