Acute kidney injury (AKI) is a common and serious syndrome affecting up to 15% of hospitalized patients and up to 50% of patients in the intensive care unit (ICU) or undergoing cardiac surgery, oncology treatment, or transplantation (1). Early diagnosis is critical so that clinicians can start appropriate management and avoid irreversible damage to the kidneys that can lead to chronic kidney disease or death.
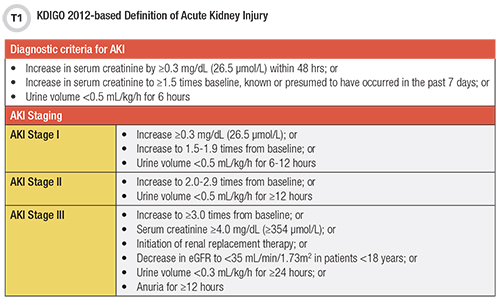
Current criteria for diagnosing and staging AKI rely on a rise in serum creatinine or drop in urine output as described in the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines (Table 1) (2). However, patients’ urine output is rarely measured, and serum creatinine is slow to react, taking 24–40 hours to increase in response to kidney injury (3). In addition, most forms of AKI do not involve the glomerulus but rather the renal tubular epithelium, so a decrease in glomerular filtration rate (GFR), as measured by creatinine or cystatin C, is not a sensitive indicator (3).
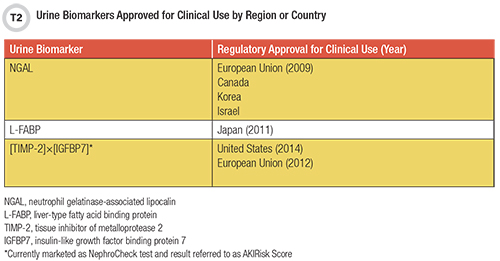
These limitations have fueled a search for a better AKI biomarker, which ideally would rapidly and specifically detect occurring kidney injury, measure prognosis, guide treatment, and improve patient outcomes. Over the last decade, several new AKI biomarkers have gained regulatory approval for human testing in different countries (Table 2), but only combined measurement of tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) [TIMP-2]×[IGFBP7] is authorized by the Food and Drug Administration (FDA) for clinical use in the United States.
Moreover, adoption of these AKI biomarkers into clinical practice has been slow due to a number of factors, leading to disparities in AKI care around the globe (4). This article will review the clinical utility of currently approved AKI markers listed in Table 2 and briefly highlight emerging approaches for the early detection of AKI.
Neutrophil Gelatinase-Associated Lipocalin
Perhaps the most studied AKI biomarker is neutrophil gelatinase-associated lipocalin (NGAL), a 25 kDa protein that was first discovered in granules of neutrophils (3). It plays important roles in the kidneys, such as induction of the genesis of tubular epithelium. NGAL also is anti-inflammatory and antiapoptotic and as a transport protein is involved in iron-trafficking.
NGAL is upregulated and released into the urine following injury to the renal tubules. We now know that NGAL is not exclusive to the kidneys: It can also be found in lungs and large intestines, and its plasma levels can also increase due to hepatic production.
Two recent meta-analyses involving more than 2,500 patients each demonstrated moderately strong areas under the curve (AUC) of 0.72–0.82 for predicting AKI (3). NGAL’s performance was stronger in studies involving children, yielding a pooled AUC of 0.90 (5). Among several biomarkers investigated in children, urine NGAL had the fastest rise postoperatively in those who developed AKI, peaking at 6 hours and remaining elevated (5).
Researchers have also investigated the predictability of long-term outcomes. Increased levels of urinary NGAL in patients with AKI were associated with 2-fold to 3.2-fold increased risk of mortality in a multi-center international study involving 1,199 adults who underwent cardiac surgery (6). On the other hand, age, female sex, urinary tract infections, and chronic kidney disease are also reported to increase urinary NGAL levels and might affect the specificity of the test (3). In addition, the availability of multiple assays and lack of standardization imply that different cutoffs are needed for every NGAL assay.
The largest barrier against implementing NGAL measurement in the U.S. is that there are currently no FDA-approved assays for this analyte. A few vendors might be selling these for research use only, which require additional considerations for validation and clinical use as laboratory developed tests.
Liver-type Fatty Acid-Binding Protein
Liver-type fatty acid-binding protein (L-FABP) is a 14 kDa member of the lipid-binding proteins superfamily and plays a crucial role in fatty acid uptake and its transfer between extracellular and intracellular membranes (3). Similar to NGAL, L-FABP is found in a variety of tissues other than the liver, like the intestine, lung, stomach, and kidney, and it is detectable in urine.
A meta-analysis of clinical studies evaluating L-FABP’s ability to predict AKI after cardiac surgery showed poor to moderate AUCs between 0.52 and 0.85 (composite AUC = 0.72) (3). Also similar to NGAL, L-FABP performed better in children with studies reporting AUCs of 0.78–0.85, but it rises later and in studies performed best when measured 12 hours post-cardiopulmonary bypass surgery (5).
Outcome studies are much more limited for L-FABP than for NGAL. However, a recent study evaluated L-FABP’s ability to predict long-term adverse outcomes in 1,119 patients being treated in a (nonsurgical) cardiac ICU and found that increased urine L-FABP (≥ 9.0 ng/mL) correlated with increased risk of all cause of death or progression to end-stage kidney disease (7). There are no FDA-approved assays for L-FABP in the U.S., limiting its adoption in clinical practice.
TIMP-2 and IGFBP7
TIMP-2 and IGFBP7—both cell-cycle regulators—are considered second-generation AKI biomarkers because they were derived using a rigorous discovery process rather than starting from a model system (3). Both markers are measured together in urine and reported as a product of both levels, [TIMP-2]×[IGFBP7], with (ng/mL)2/1000 as the unit.
This combined measurement method was the first FDA-approved test for assessing the risk of developing AKI. It was approved in 2014 for predicting the development of moderate to severe AKI (KDIGO stage 2 and 3) within 12 hours from sample collection. At the present time, standardization is not a problem, because Astute Medical (now owned by bioMérieux) is the only company that produces and markets this test under the name NephroCheck with the result labeled AKIRisk.
In early validation studies, urinary [TIMP-2]×[IGFBP7] outperformed all other markers—including urinary and plasma NGAL, plasma cystatin C, and urine L-FABP—for the development of moderate to severe AKI within 12 hours, with an AUC of 0.80. This test has since been studied in over 1,800 critically ill patients in different settings with promising outcomes (8).
However, the performance of the test in studies has varied widely depending on the cutoff, testing time, and diagnostic criteria used. First, the recommended 0.3 (ng/mL)2/1000 cutoff had poor specificity and generated a significant number of false positives, while a threshold of 2.0 (ng/mL)2/1000 yielded almost 100% specificity but poor sensitivity. This is not surprising considering that the thresholds fall within the reference intervals, which were reported to be 0.04–2.22 (ng/mL)2/1000 from 750 subjects by a large multi-center study (9). Second, the AUCs were best when measured 3–12 hours from cardiopulmonary bypass surgery, ICU admission, chemotherapy administration, or other procedures with high risk for AKI development.
Lastly, studies that evaluated the performance of [TIMP-2]×[IGFBP7] for the development of Stage 1 AKI—for which the test was not approved—reported poorer performance, as expected. However, urinary [TIMP-2]×[IGFBP7] has not been studied as well in pediatric populations, and clinical labs are cautioned against implementing this test in patients with low risk for developing AKI due to its suboptimal specificity (8). Therefore, optimization of different cutoffs and collection times for specific populations is needed prior to implementation.
Overall, lack of widespread adoption might be attributed to this test’s suboptimal specificity at the recommended 0.3 (ng/mL)2/1000 cutoff, limited performance studies outside of ICU or perioperative settings, and lack of positive impact on patient outcomes. Clearly [TIMP-2]×[IGFBP7] is no kidney troponin, and future studies should examine if combining it with clinical examinations, symptoms, or other biomarkers will improve its diagnostic accuracy.
Nevertheless, this test’s clinical utility has been demonstrated in very specific populations and is already recommended for use in certain practice guidelines, most notably for perioperative care in cardiac surgery (4). However, both the National Institute for Health and Care Excellence (NICE) in the U.K. and the AACC Academy in the U.S. (in press) state that there is not enough evidence to recommend its routine use (10). They recommend additional research to assess [TIMP-2]×[IGFBP7]’s clinical effectiveness as part of care bundles and its effect on clinical outcomes.
Novel Markers and Tools
The clinical utility of other AKI biomarkers can be further subclassified into biomarkers of tubular injury, tubular function, kidney inflammation, and adaptive repair and fibrosis (11). A detailed discussion on these research markers is beyond the scope of this article, but interested readers will find an excellent summary by Zhang et al. (11).
While some of these other markers hold promise, notably their performance in clinical trials has varied significantly. This variability might trace back to the use of research assays from different vendors and the lack of sufficient information in reported studies on the assay used or the epitope of antibody targets, among other reasons (12). Therefore, researchers and assay manufacturers need to provide in their reports detailed assay information, validation data, and preanalytical data.
Another exciting development in this space came from a collaboration between clinicians and machine-learning researchers from a Google subsidiary in the U.K. called DeepMind. This research, reported by Tomasev et al. in Nature, leveraged data from more than 700,000 individuals across 1,239 healthcare facilities in the U.S. Veterans Affairs database to create a computational model that predicts the development of AKI in 48 hours (13). The reported AUC was 0.92, eclipsing the best performing blood and urine biomarkers studied thus far.
This research effort employed an algorithm with impressive complexity, including 620,000 variables as inputs to the model. A major downside is that any one of these inputs can “break” easily, as can happen when, for example, a test name is changed or a lab migrates laboratory information systems (14). As a result, thorough validation and quality management plans are desperately needed before artificial intelligence can be safely implemented in clinical medicine (15).
Conclusion
Over the last decade, we have seen a rise in the number of AKI biomarker assays gaining regulatory approval for clinical use, with some even making it into clinical practice guidelines. Current research is still focused on understanding the different roles that current and emerging biomarkers play in AKI diagnosis, management, and prognosis.
Alas, the hunt for the elusive kidney troponin continues. If recent reports are any indication, then perhaps it has always been in front of our eyes. We just lacked the “intelligence” to recognize it.
Joe M. El-Khoury, PhD, DABCC, FADLM, is an associate professor of laboratory medicine at Yale University School of Medicine and director of the clinical chemistry laboratory and clinical chemistry fellowship program at Yale-New Haven Health in New Haven, Connecticut. +Email: [email protected]
References
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394:1949-64.
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International 2012;2:1-141.
- Srisawat N, Kellum JA. The Role of Biomarkers in Acute Kidney Injury. Crit Care Clin 2020;36:125-40.
- Lieske JC, Kashani K, Kellum J, et al. Use of Biomarkers to Detect and Manage Acute Kidney Injury: Has Progress Stalled? Clin Chem 2020;66:271-6.
- Sandokji I, Greenberg JH. Novel biomarkers of acute kidney injury in children: an update on recent findings. Curr Opin Pediatr 2020;32:354-9.
- Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 2014;25:1063-71.
- Naruse H, Ishii J, Takahashi H, et al. Urinary Liver-Type Fatty-Acid-Binding Protein Predicts Long-Term Adverse Outcomes in Medical Cardiac Intensive Care Units. J Clin Med 2020;9.
- Fan W, Ankawi G, Zhang J, et al. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin Chem Lab Med 2019;57:567-76.
- Chindarkar NS, Chawla LS, Straseski JA, et al. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7][TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin Chim Acta 2016;452:32-7.
- National Institute for Health and Care Excellence. Tests to help assess risk of acute kidney injury for people being considered for critical care admission (ARCHITECT and Alinity i Urine NGAL assays, BioPorto NGAL test and NephroCheck test). (Accessed September 29, 2020, at https://www.nice.org.uk/guidance/dg39/chapter/1-Recommendations.)
- Zhang WR, Parikh CR. Biomarkers of Acute and Chronic Kidney Disease. Annu Rev Physiol 2019;81:309-33.
- Bunch DR, El-Khoury JM. Emerging Biomarker of Kidney Disease: suPAR or Subpar? Clin Chem 2018;64:1545-7.
- Tomasev N, Glorot X, Rae JW, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019;572:116-9.
- Wilson FP. Machine Learning to Predict Acute Kidney Injury. Am J Kidney Dis 2019.
- Schulz WL, Durant TJS, Krumholz HM. Validation and Regulation of Clinical Artificial Intelligence. Clin Chem 2019;65:1336-7.