Quality Control Materials: The Classic Make or Buy Decision
All laboratory determinations have some degree of uncertainty. Total error is the sum of systematic and random errors, expressed quantitatively as bias and imprecision, and assessing accuracy and reproducibility, respectively. Regulatory entities and good laboratory practices require evaluating an assay’s performance characteristics before using it to test patient samples. As such, total error is both quantified and accepted when it falls within the total allowable error for the test and its intended medical use. To evaluate whether a method continues meeting its medical and analytical goals, laboratories monitor the performance of the test using a quality control (QC) plan that alerts the lab of problems with potential effects on patient test results.
Labs face challenges in their routine statistical QC despite a wealth of theoretical knowledge on initiating and maintaining a QC program. The Clinical and Laboratory Standards Institute (CLSI) guideline, Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions (C24-A3, Third Edition), lists several approaches for labs to improve how they use statistical QC. These include: planning QC based on the performance and quality requirements for the test; defining an analytical run tailored to both the method and laboratory operations; and implementing procedures for QC and out-of-control situations. Planning QC starts with the selection of appropriate control materials and extends to the frequency, the number of control samples to run, and acceptability rules. CLSI C24-A3 explains the mathematical approach behind and selection criteria for the latter concepts.
Selecting appropriate and adequate QC materials—an absolute must—should occur at the beginning of the planning process. Quality monitoring of laboratory tests relies heavily on using QC materials at certain pre-defined concentrations and time intervals, which challenge the system according to pre-established acceptability rules. This makes the quality of the QC materials themselves a cornerstone of successful quality monitoring programs.
What Constitutes Good?
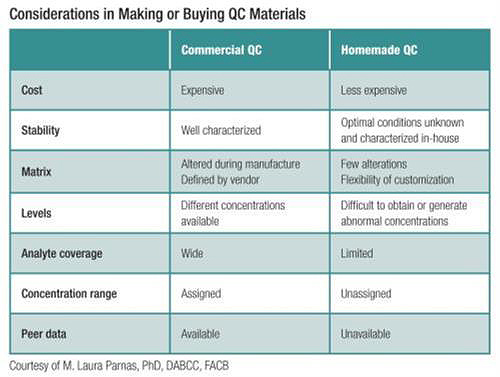
Good QC materials have qualities that not only put the system through its paces but also provide valid information on the system’s performance. QC materials of composition (or matrix) equivalent to patient samples minimize possible matrix effect interferences. The best OC materials also are stable with expiration dates at least one year into the future and have well-characterized shelf life stability after their original storage conditions change whether through thawing, reconstitution or storage in an opened bottle.
Labs need to evaluate common sources of QC material variability up front and mitigate this variability by statistically assessing new lots of QC and by standardizing procedures for handling the materials. Commercial QC materials—available for most laboratory tests—often fulfill these desired qualities. Conveniently, one solution usually contains many related analytes, such as for multiple cardiac markers, and includes a range for the expected concentration of each measurand. For those materials termed “assayed,” the concentration range is stratified according to the analytical principle and even assay manufacturer. Regardless, regulations and good laboratory practice require running the materials for at least 20 days to establish baseline statistical characteristics (mean and standard deviation).
CLSI C24-A3 establishes that “if commercial quality control materials are not available, the laboratory may prepare and aliquot patient pools for this purpose.” As outlined above, use of commercial QC poses several advantages over homemade preparations, including access to peer data. Even so, in some circumstances it may be beneficial or even an absolute necessity to prepare QC materials in-house. The need to do so might arise if QC materials are not commercially available for the assay or matrix being tested, if the materials are not available at one or more clinically relevant concentrations, or if the lab could save costs without sacrificing the quality of the materials. In-house preparation of QC materials is particularly common for laboratory-developed tests, such as those performed using mass spectrometry. Accordingly, the CLSI guideline, Liquid Chromatography-Mass Spectrometry Methods (C62-A, First Edition) has a section on quality assurance and QC thoroughly covering considerations related to the QC plan.
The Logistics Challenge
Labs certainly have the wherewithal to prepare homemade QC, as long as they are willing to take care of all the associated logistics, which may be labor intensive initially. Labs that take this route need to identify the source of and desired concentrations for the analyte in question. Patient pools may be appropriate particularly if already present in the test matrix; otherwise, standard materials are suitable as long as the lot used for QC preparation is not the same as the lot used for standards. The latter may facilitate preparation of multi-analyte QC.
Labs taking this approach also need to consider the matrix and stability of the QC materials. For analytes not normally present in samples—like drugs-of-abuse—leftover samples characterized as analyte-free may be used. For other applications, labs would obtain commercial urine or plasma, which adds cost but ensures QC reliability.
Work with the materials doesn’t stop once they’re prepared. Labs also need to characterize their storage and stability criteria through a well-documented study that includes acceptability criteria, evaluates stability at different temperatures during short- and long-term storage, and analyzes the effect of temperature changes if the QC will be subjected to these (e.g. freeze-thaw cycles). As is the case with commercial QC, labs need to establish baseline statistics for each analyte. One option is to collaborate with companies that offer custom QC. If financially viable, this approach could spare the laboratory from the preparation and stability characterization steps. Whether they ultimately employ commercial or in-house QC materials, each laboratory needs to evaluate the approach that best suits its needs and ensures a robust quality program.
Jessica M. Colón-Franco, PhD, DABCC, is an assistant professor of pathology at the Medical College of Wisconsin in Milwaukee, and director of clinical chemistry and point-of-care at Wisconsin Diagnostic Laboratories and Froedtert Hospital. +Email: [email protected]