According to the Centers for Disease Control and Prevention (CDC) as of October 4, 2022, more than 1,055,192 COVID-19 deaths were reported since the onset of the pandemic. COVID-19 has contributed to significant increases in hospital-acquired infections (HAI) that may lead to sepsis from central-line-associated bloodstream infections (BSIs), catheter-associated urinary tract infections, ventilator-associated events, and select surgical site infections. The four most common causative pathogens for these infections include S. aureus, E. coli, C. albicans, and P. aeruginosa1,2 and contribute to longer hospital stays, and longer Intensive Care Unit (ICU) stays.
A recent study in The Lancet revealed that 11 million people globally died from sepsis in 2017 , and the CDC reports nearly 270,000 Americans die because of sepsis each year. Despite how common and deadly sepsis is, there is a lack of adequate diagnostic technologies to identify sepsis-causing infections fast enough to impact clinical decisions and outcomes. Any delay in time to detection has implications for patients and hospitals, including patient outcomes, antimicrobial resistance, hospital costs, and healthcare economics.
Current standard of care for detecting bloodstream infections
In patients without a confirmed source of infection, standard guidelines recommend physicians initiate patients suspected of BSIs on broad-spectrum therapy. Broad-spectrum or empiric therapy treats a wide range of organisms but does not necessarily target the specific pathogen causing the patient's infection. Blood cultures have long been regarded as the gold standard for diagnosing BSIs, but they can take time to deliver results. A healthcare team may have to wait several days to confirm the cause of a BSI. Subsequent susceptibility testing is often utilized to determine which therapy will be most effective in fighting the specific pathogen, especially those that are resistant to certain antimicrobials.
Antimicrobial resistance (AMR) occurs when bacteria, viruses, fungi, or parasites change over time and no longer respond to antimicrobials, making infections harder to treat and increases the risk of the spread of disease, severe illness, and death. The Clinical Microbiology Reviews reports that acquiring an antimicrobial-resistant infection caused by the ESKAPE pathogens — Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species reduces viable treatment options for serious infections, heightens the burden of disease, and amplifies mortality rates because of ineffective treatment.
Limitations in the current standard of care
While waiting for blood culture results, patient conditions may continue to deteriorate if the broad-spectrum antimicrobial therapy is not effective against the underlying BSI. As a result of inappropriate or delayed antimicrobial therapy, patients are at risk of developing potentially life-threatening complications such as sepsis and septic shock.
Time is of the essence when combatting BSIs and sepsis, making the lengthy nature of blood cultures problematic. A study in Critical Care Medicine indicates that for every hour of delay in time to appropriate therapy in a septic shock patient, survival decreases by 7.6%. Blood cultures detect bacteremia in just 50% of patients who have documented severe sepsis according to a study in JAMA: The Journal of the American Medical Association. The time to identification and relative low sensitivity of blood cultures may prove a hindrance to early effective treatment of bloodstream infections. With millions of people impacted by sepsis every year across the globe, it is critical for physicians to have access to the most effective diagnostic tools that enable prompt initiation of targeted therapy.
Besides the potential for poor patient outcomes, this diagnostic dilemma can have additional implications for the healthcare ecosystem. Overuse of unnecessary antimicrobial drugs is costly and is a key risk factor associated with the development and spread of antimicrobial resistant organisms. Furthermore, delays in targeted treatment can lead to an increased length of stay in the hospital and use of facility resources. In fact, sepsis accounts for more than $62 billion in annual costs, making it the most expensive condition to treat in the entire U.S. healthcare system.
Culture-independent diagnostics
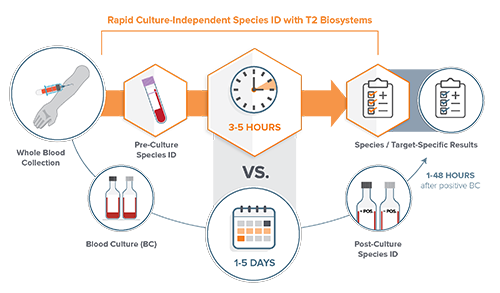
Fortunately, there are new diagnostic technologies that do not rely on positive blood cultures for species identification. Culture-independent diagnostics such as the T2Bacteria Panel and the T2Candida Panel from T2 Biosystems, are the only FDA-cleared diagnostic assays for the detection of sepsis-causing bacterial and fungal pathogens directly from whole blood. Results from the T2Bacteria and T2Candida Panels are available within three to five hours of the first blood draw. By providing quick and accurate results, the panels enable clinicians to target therapy faster for patients suspected of sepsis, often before the second dose of antimicrobials is administered. This can lead to better patient outcomes, improved antimicrobial stewardship, and reductions in length of hospital and ICU stays.
Studies show that direct-from-whole-blood technology provides a faster turnaround time (TAT) than blood cultures and has a high sensitivity and specificity (greater than 90%). In a prospective, single center study of newly admitted patients to an infectious disease unit, the T2Bacteria Panel detected more pathogens than blood culture. Time from admission to targeted antibiotic therapy was, in median hours, 6.6 hours with T2Bacteria Panel vs. 77.7 hours (about 3 days) with blood cultures. Likewise, a study in the Journal of Emergency Medicine states that the T2Bacteria assay detected 25% more positives associated with infection, and on average identified the species 56.6 hours (about 2 and a half days) faster compared to blood cultures.
Enhancing the standard of care for sepsis
Culture-independent diagnostics have the potential to enhance the standard of care for sepsis by making a positive impact on patients and institutions through earlier species identification, faster-targeted therapy, and reduced length of hospital and ICU stays. The COVID-19 pandemic has forever changed the face of medicine and has emphasized the value of reliable diagnostic results. 70% of today's medical decisions are dependent on laboratory results , it’s imperative that they are available fast enough to impact clinical decisions and outcomes for physicians and patients. As a healthcare community, we must continue to innovate and utilize technologies that have the potential to impact the management of serious conditions such as sepsis.
References
- Centers for Disease Control and Prevention. COVID-19 Mortality Overview. 16 05 2022. https://www.cdc.gov/nchs/covid19/mortality-overview.htm. 12 10 2022.
- Weiner-Lastinger, Lindsey M. "The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network." Infection Control and Hospital Epidemiology (2021).
- Rudd, Kristina, “Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study.” The Lancet. 2020
- Centers for Disease Control and Prevention. “What is Sepsis?” 2020. https://www.cdc.gov/sepsis/what-is-sepsis.html
- Donald M. Yealy, MD. "Early Care of Adults With Suspected Sepsis in the Emergency Department and Out-of-Hospital Environment: A Consensus-Based Task Force Report." Annals of Emergency Medicine (2021).
- Oliveira, David M. P. De. "Antimicrobial Resistance in ESKAPE Pathogens." Clinical Microbiology Reviews (2020).
- Kumar, Anand. "Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock." Critical Care Medicine (2006).
- Brun-Buisson, C. "Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis." JAMA (1995).
- Buchman, Timothy G. PhD, MD. "Sepsis Among Medicare Beneficiaries: 3. The Methods, Models, and Forecasts of Sepsis, 2012–2018." Critical Care Medicine (2020).
- Giannella, Maddalena, et al. Expert Review of Medical Devices, 2021
- Seitz, Tamara, et al. “Evaluation of the clinical impact of the T2MR for the diagnosis of blood stream infections.” Poster Presentation, IDWeek. 2019.
- Voigt C, et al. “The T2Bacteria Assay Is a Sensitive and Rapid Detector of Bacteremia That Can Be Initiated in the Emergency Department and Has Potential to Favorably Influence Subsequent Therapy.” J Emerg Med. 2020 May;58(5):785-796. doi: 10.1016/j.jemermed.2019.11.028. Epub 2020 Jan 23. PMID: 31982197.
- Centers for Disease Control. Strengthening Clinical Laboratories. https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html
Industry Insights articles are created and paid for by advertisers. The views expressed in these articles do not necessarily represent AACC’s views, and their inclusion in CLN is not an endorsement by CLN or AACC.