Medical oncologists strive daily to provide the best cancer care for each of their patients. However, the rapid rate of practice-changing developments makes keeping up to date a significant challenge.
Furthermore, a recent report from the American Society of Clinical Oncology outlines trends that could worsen the caseload burden for practicing medical oncologists.. These include lengthened cancer patient survival and a decreased number of medical oncologists entering the United States workforce between 2009 and 2018 (1).
Understanding the Problem
The dueling issues of high medical oncologist caseload and the expanding complexity of practical cancer care provide an impetus to avoid compromising patient care. This includes meeting success metrics, such as achieving National Comprehensive Cancer Network (NCCN) guidelines or similar evidence-based consensus strategies. A recent retrospective study of 1,497 patients with metastatic colorectal cancer from 23 practices across the U.S. (2013–2017) assessed how often the molecular tumor testing that patients received met NCCN guideline recommendations (2). Surprisingly, testing rates for guideline-aligned RAS, BRAF, and microsatellite instability/mismatch repair deficiency were 41%, 43%, and 51%, respectively.
Patients were more likely to have guideline-aligned testing if they were female, treated at an academic center, and diagnosed with de novo metastatic disease (2). Of the 177 patients (12% of the cohort) who received anti-EGFR therapy (primarily panitumumab or cetuximab), only 50 (28%) had undergone guideline-aligned biomarker testing (2). This is especially concerning because it means that the patient could be exposed to the systemic toxicity risk from anti-EGFR therapy without benefiting from the effect of anti-EGFR therapy in the presence of a KRAS or NRAS driver mutation. These results illustrate one example, in a common single cancer type, where underutilized genetic testing led to missed opportunities for guideline-supported therapies based on absent biomarker-directed therapeutic considerations.
Precision medicine stratifies patients according to predictive biomarkers associated with sensitivity to targeted cancer therapies. The impact of this approach on cancer treatment outcomes continues to grow annually. The “2020 Personalized Medicine Report” released by the Personalized Medicine Coalition reported that between 2016 and 2020, the number of personalized medicines grew from 132 to 286 (a 113% increase) (3). As of 2018, 39% of cancer therapy clinical trials employed biomarker-based inclusion criteria to stratify patients likely to respond to therapy (up from 25% in 2010). Targeted biologic therapies now represent over 90% of the total oncology development pipeline (4).
A recent study by the University of Texas MD Anderson Cancer Center's Precision Oncology Decision Support (PODS) team compared their genomic interpretations of actionability and related therapeutic options against those made by the physicians of treated patients (5). The authors found that while physicians were often able to detect hallmark hotspot alterations in their patient’s molecular results, physicians frequently interpreted alterations as nonactionable when the PODS genomic annotators classified them as actionable or potentially actionable. Discordant alteration interpretations and determined actionability in some cases represented missed therapeutic opportunities for patients (5).
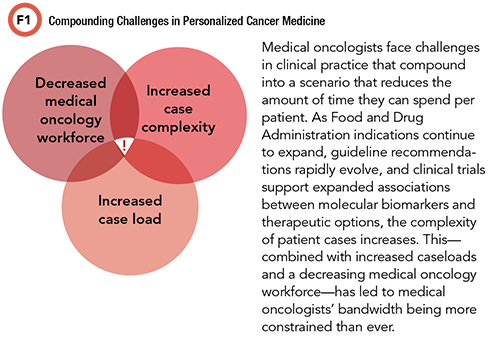
Outlining the Need
Medical oncologists need reliable and concise strategies to clarify therapeutic recommendations that consider patient-specific characteristics. Correlating somatic alterations to a patient’s clinical scenario is a time-consuming endeavor that requires continual updating to reflect the rapidly evolving information in the field of oncology. Contextualizing and sequencing somatic alteration interpretations with potential treatment options is prohibitively time-intensive for medical oncologists, a trend that will intensify even more as caseloads increase.
High patient volumes, limited time per patient, and the complexity of using somatic molecular reports are all likely contributors to why less than half of metastatic colorectal cancer patients received NCCN guideline-recommended testing and may have missed potentially beneficial targeted therapy (2). In cases in which a patient harbors potentially actionable alterations or variants of unknown but possible significance, molecular tumor boards and thorough literature searches for supporting data on the function of the alteration and related therapy options are required. Precision medicine experts can support medical oncologists by performing such interpretations and therapy considerations in a distilled consult for individual patients.
Medical oncologists often depend on a specialized pathologist to interpret and report relevant diagnostic findings. Similarly, medical oncologists with imaging needs can rely on a specialized radiologist. Conversely, in most cases, when a medical oncologist orders a molecular cancer panel, they are left to review and interpret the therapeutic application of results, often with no formal training on interpreting genomic alterations of lesser-known or unknown significance into a clinical context.
Finding the Solution
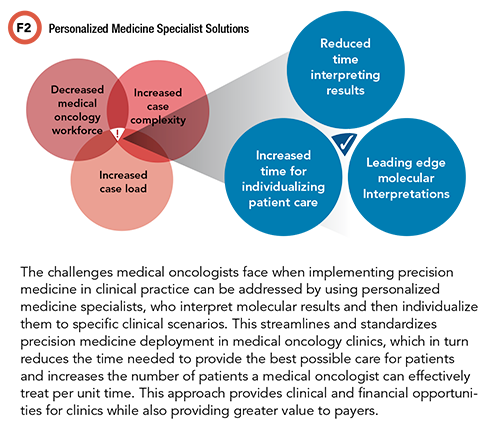
The presence of a medically focused precision medicine consultative service has shown proven value in the context of the current complexity and time constraints that medical oncologists face. This can take the form of an in-house molecular tumor board-style service (often a mix of pathology, medical oncology, and pharmacy) or partnership opportunities with clinical consultative companies.
This approach will help medical oncologists reduce the amount of time spent interpreting somatic alterations and ensuring that interpretations are up to date with the most recent data relevant to individual cases. In turn, this will standardize and improve clinical care. The reporting from such a consultative service is also of high impact in letter of medical necessity discussions, where the goal is insurance company support for precision oncology options. By reducing the time burden spent per patient on result interpretation and therapeutic considerations, improved time efficiency may also strengthen revenue.
Conclusion
Utilizing a precision medicine consultative service to contextualize somatic test results relevant to individual cases, medical oncologists can combat the underutilization of guideline-aligned genomic testing and ensure a clear strategy for therapy options and the best available care for patients.
References
- 2020 Snapshot: State of the oncology workforce in America. JCO OP 2020;17:30.
- Gutierrez ME, Price KS, Lanman RB, et al. Genomic profiling for KRAS, NRAS, BRAF, microsatellite instability, and mismatch repair deficiency among patients with metastatic colon cancer. JCO Precis Oncol 2019;3:1–9.
- Personalized Medicine Coalition. The personalized medicine report: An annual overview of the field. http://www.personalizedmedicinecoalition.org/Resources/The_Personalized_Medicine_Report_An_Annual_Overview_of_the_Field (Accessed March 2021).
- IQVIA Institute. Global oncology trends 2019. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019 (Accessed March 2021).
- Brusco LL, Wathoo C, Mills Shaw KR, et al. Physician interpretation of genomic test results and treatment selection. Cancer 2018;124:966–972.
Ryan S. Nelson, PharmD, is a senior pharmacy consultant in the department of consultative services at ARUP Laboratories, Salt Lake City. +Email: [email protected]
Howard L. McLeod, FASCO, FCCP, is the medical director of PharmD, precision medicine at the Geriatric Oncology Consortium in Tampa, Florida. +Email: [email protected]