In 2019, what do we know about the future for quantitative, open-access liquid chromatography (LC)-mass spectrometry (MS) testing with laboratory developed tests (LDTs) in hospital laboratories?
Market analyses for diagnostic LC-MS testing predict robust expansion, with precision medicine applications and the search for new biomarkers driving demand (1,2). In contrast, forecasts for U.S. hospital laboratory finances are uniformly negative because of decreased payments from Medicare, due to the Protecting Access to Medicare Act and workforce shortages (3,4). Where in this spectrum from optimistic growth to pessimistic budget constraints does the reality lie?
Cost per reportable test calculations for clinical laboratories are most favorable when there are high sample volumes per test and extensive automation (5). For the hormone and drug tests usually performed with LC-MS, the sample numbers per test are small at individual hospitals. But these small workloads become significant once aggregated at reference laboratory esoteric testing sites. Economies of scale therefore generate favorable cost per reportable outcomes for LC-MS at reference laboratories.
In contrast, hospital LC-MS typically has low workload per high complexity assay and minimal automation—a perfect storm that increases cost per reportable test (6). Moreover, the labor cost for hospital LC-MS tests is typically much higher than for immunoassays on automated, high throughput systems (6). The inherent technical and process challenges of open access instruments and LDTs remain the norm for LC-MS (6).
LC-MS deployment in chemistry departments has been slow compared with how rapidly microbiology sections in the same hospitals have adopted matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS (7). How has the balance between technical scope (high score for LC-MS), ease of use (low score for open access, LDT LC-MS), and clinical value (hotly debated) tilted at different sites to promote or limit LC-MS use?
To address these questions, CLN surveyed a sample of hospital LC-MS laboratory directors about their use of this exceptional technology. For this discussion, health system core laboratories and regional laboratories are considered hospital laboratories. We focus on hospital use because the business case for LC-MS use at reference laboratories already is well established. Eighteen of the 33 invitees responded. A summary and discussion of the survey responses follows.
The full list of identified survey respondents, their institutions, and job titles can be seen in the online version of the article at www.myadlm.org. Survey participants had the option to be listed as contributors or not. Three participants elected to remain anonymized.
Balancing LC-MS and Alternative Methods
The first question in our survey asked what factors influence the decision to use LC-MS versus other technologies for measuring an analyte (Figure 2).
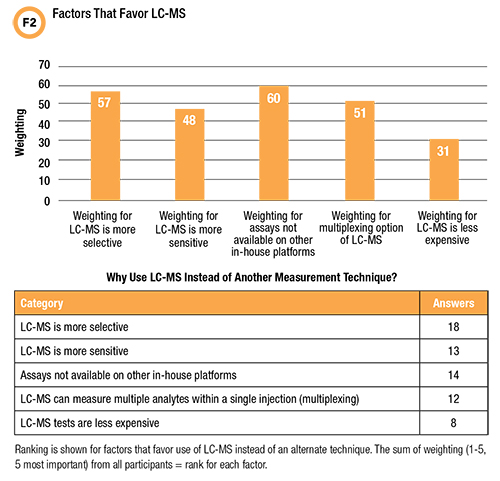
The only factor chosen by all respondents was greater selectivity. But when choices are ranked for importance, the highest rated reason for using LC-MS is revealed as the lack of an assay on another in-house platform. The lowest frequency (44%) and ranking in the survey was for LC-MS tests being less expensive.
These answers suggest that an LC-MS test menu emphasizing analytes that can be tested only by using LC-MS might be a stronger strategy for justifying new or expanded LC-MS use in hospital laboratories than trying to compete against immunoassay on cost or clinical value.
The greater selectivity of LC-MS compared with other measurement techniques is uncontested: The challenge has been convincing clinicians and administrators that greater selectivity alone has sufficient value—notably better clinical outcomes or sufficient global health system cost savings—to justify LC-MS when a less costly, more automated measurement technique is available (6).
Moving LC-MS Testing Back to Another Platform
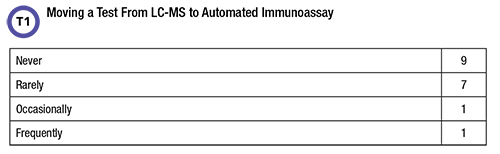
The second part of the initial question asked respondents whether they’d moved a test from LC-MS to an alternative platform (Table 1).
The survey also asked about the reasons behind the switch from LC-MS to another technology. The options were: Workload, insufficient throughput using LC-MS; Complexity, automated immunoassay is simpler, more results can be reported/technologist; Cost, switched when automated immunoassay cost is less than or equal to LC-MS cost; Quality, switched when automated immunoassay quality became acceptable; Random access, workload decreased and LC-MS batch mode was too expensive, while random access automated immunoassay was feasible.
Fifty percent of respondents replied that an LC-MS test had been switched to a different technology at their laboratory although most indicated it was a rare event. Reasons for a technology switch are shown in Figure 3.
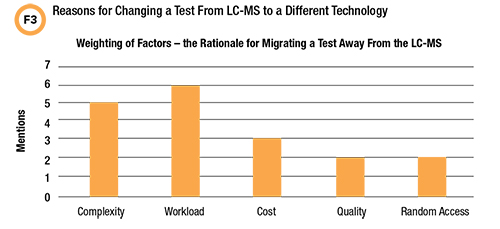
It’s important not to over-interpret the limited data on this question. But for hospital laboratories in 2019, the feasibility rationale for preferring automated immunoassay may be more compelling than anticipated unless the clinical justification for using LC-MS is very strong. Note that high complexity and limited throughput of LC-MS were the most common reasons for migrating tests to other platforms.
What Does the Future Hold?
The second question in the survey dealt with respondents’ view of the future for LC-MS testing in their institutions (Table 2).
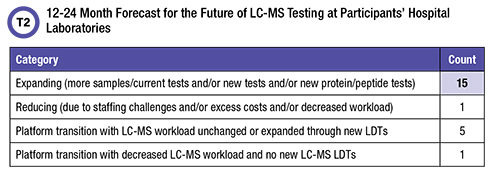
Of those respondents who reported that they anticipate expansion, 14 included implementing new LDTs in their plans. New protein/peptide assays are a factor in the expansion outlook for only three participants. Four respondents included transitioning tests from LC-MS to other platforms in their plans, as well as expanding LC-MS LDT workload. And three contributors plan to transition 25-OH vitamin D testing from LC-MS to automated immunoassay. The one hospital laboratory that expects to reduce LC-MS testing selected labor challenges as the reason.
The majority (83%) of participants anticipate expanding their hospital LC-MS testing service. At the same time, it appears that transition of some testing from LC-MS to less complex, more automated platforms is a reality. One recovery strategy for LC-MS laboratories that lose workload to other in-house platforms could be to aggressively develop new assays. A high percentage of contributors (78%) did indeed answer that they are planning growth through offering new tests.
Sites that promote new assay development will be well prepared if the model for sustaining LC-MS practice in hospitals turns out to be a continuous recycling of the test menu. By this we mean a laboratory first develops new LC-MS tests to optimize patient care, expecting that analytes which become the standard of care will eventually transition to automated platforms. As these tests make their way to automated platforms, the lab already would be developing another round of new LC-MS LDTs.
Open access LC-MS LDTs are uniquely suited for timely implementation of new assays (6). This niche would play to the inherent strengths of hospital LC-MS—expertise in LDT method development, laboratory-clinical team collaboration, high specificity, multiplexing capability, and short turnaround times compared with outsourcing (6,8). In contrast, competing with automated immunoassay or reference laboratory LC-MS highlights the downsides of hospital-based open access LDT LC-MS: lower throughput, less automation, higher labor costs, and small batch workflows.
Looking ahead toward potential growth of LC-MS testing in hospital laboratories, a follow-up to the second question in the survey asked what subspecialty areas of LC-MS testing participants would add or expand to deliver value to their healthcare systems (Figure 4).
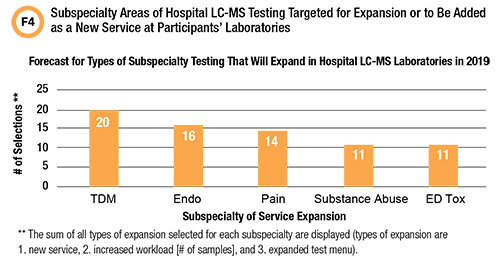
Fifteen contributors (83%) chose a new therapeutic drug monitoring (TDM) service, or enhancement of an existing TDM service, as a means to expand LC-MS. Hospital laboratories have the advantage of shorter TDM turnaround times, allowing for more rapid dose optimization (9). LC-MS testing is often the only option available for therapeutic monitoring of new drugs or traditional drugs for which interest in TDM is new (9).
None of the respondents in the survey mentioned novel protein/peptide or small molecule biomarkers as a preferred means to expand hospital LC-MS testing.
The Question of Automation
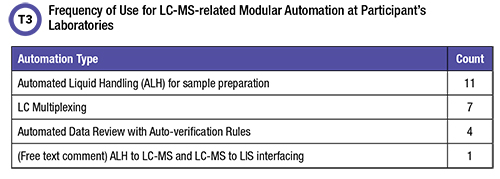
A third question in the survey tackled automation, asking respondents what was now in use to automate their LC-MS testing. A follow-up also asked if a closed, fully automated, FDA approved LC-MS system became available, would they transition all LC-MS LDTs to this platform and discontinue open access LC-MS—or would they retain open access LDT LC-MS capability with new LDTs and use both systems.
Table 3 indicates that existing modular automation which can decrease labor costs and increase throughput is underutilized. Of course, capital equipment funding and in-house technical expertise are a challenge. Ten participants (56%) said they would retain their open access LDT LC-MS and develop new LDT, seven made no selection, and one chose replacement.
An FDA approved system with the exquisite selectivity of LC-MS but similar ease of use to automated immunoassay offers the best of both worlds—lower labor costs, no need for special expertise, random access functionality, avoiding the extra work of LDTs, and mitigating the insufficient specificity of some immunoassays.
What added value does open access LC-MS capability provide such that 56% of participants would choose to have both types of LC-MS instruments in their laboratories? Test menu is certainly a consideration. Closed, FDA approved LC-MS systems may not have the full menu of assays now available as LDTs. But for sites with the necessary resources, the most attractive feature may be that a very powerful analytical tool and local control of test menu and assay performance provide unique opportunities to optimize and customize patient care (6,8).
What Will Drive Change in Hospital Laboratories?
A fourth and final question in the survey asked respondents which factors are most important for the future of LC-MS testing at their institutions (Figure 5). Twelve participants chose both insufficient staffing and more regulation as the major factors that could compromise LC-MS testing at their site. Only five chose novel assays as having the potential to expand their LC-MS operations.
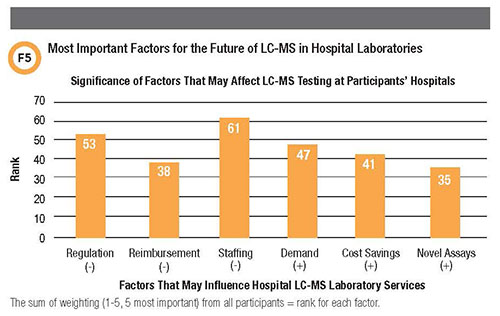
Overall, the responses indicate more concern about technical capability—regulation and staffing—and less about finances, such as poor reimbursement, cost savings, or increased demand for testing.
Conclusions
These responses present a more nuanced picture of the future for hospital LC-MS testing than traditional wisdom would indicate. While a majority of participants predicted expansion of their service, several already have, or plan to, transition at least one LC-MS LDT to a non-LC-MS platform, and one site will discontinue their open-access LDT LC-MS diagnostic service.
Importantly, respondents’ concern about staffing shortages indicate that LC-MS training needs to expand, with an emphasis on method development and validation skills (10).
This survey suffers from small sample size and selection bias in the invitations to participate. Despite these limitations, we hope to stimulate discussion in the LC-MS community on this topic and provide food for thought to all hospital LC-MS laboratory directors and those considering a new LC-MS implementation.
Judith Stone, MT(ASCP), PhD, DABCC, is a clinical laboratory scientist based in San Francisco, California. +Email: [email protected]
ACKNOWLEDGMENT
The author would like to thank survey participants for contributing their time and expertise and the CLN Focus on Mass Spectrometry Editorial Board for productive discussion on the future of diagnostic LC-MS: Donald Mason, Frederick Strathmann, Paul Jannetto, and Yan Victoria Zhang.
References
- Strategic Directions International. LDTs drive clinical mass spectrometry. https://strategic-directions.com/ldts-drive-clinical-mass-spectrometry/(Accessed September 2019).
- Digital Journal. Clinical mass spectrometry market to surge past US$8.7 billion by 2025 due to widened application area and new technological advancements. http://www.digitaljournal.com/pr/3508745 (Accessed September 2019).
- Scott K. Survival of the fittest in a post-PAMA world. Clinical Laboratory News 2018;44(2):22-4.
- American Society for Clinical Laboratory Science (ASCLS). Addressing the clinical laboratory workforce shortage, position paper of ASCLS, approved August 2, 2018. https://www.ascls.org/position-papers/321-laboratory-workforce/440-addressing-the-clinical-laboratory-workforce-shortage (Accessed September 2019).
- Data Innovations. Cost per test, overview. http://www.datainnovations.com/sites/default/files/EE_Help/EE12/Content/CPT/costpertestoverview.htm (Accessed September 2019).
- Vogeser M, Zhang VY. Understanding the strategic landscape surrounding the implementation of mass spectrometry in the clinical laboratory: A SWOT analysis. Clin Mass Spectrometry 2018;9:1-6.
- Paxton A. MALDI in microbiology: Set to stun? CAP Today 2015.
- Zhang VY, Cotton S. New tests, new metrics for success, how labs in multidisciplinary teams enhance mass spectrometry’s institution-wide value. Clinical Laboratory News 2019;45(4):24-7.
- Shipkova M, Svinarov D. LC-MS/MS as a tool for TDM services: Where are we? Clin Biochemistry 2016;49:1009-23.
- Stone JA, Fitzgerald RF. Liquid chromatography–mass spectrometry education for clinical laboratory scientists. Clin Lab Med 2018;38:527–37