Sometimes laboratory results just don’t make sense: an extremely elevated serum amylase measurement in a patient with no abdominal pain or related symptoms, or a thyroid-stimulating hormone (TSH) value that is 12 times higher than the reference interval in a patient with no changes in free thyroxine or signs of hypothyroidism. Or maybe the results don’t seem reasonable in the context of other laboratory results—why would the aspartate aminotransferase (AST) be 40 times higher than the alanine aminotransferase (ALT)? Why would amylase be consistently elevated without any observed elevation in lipase? Helping to interpret results in situations such as these is a big part of our job as laboratorians.
Troubleshooting the analytical measurement or the effects of preanalytic factors on these and other analytes is commonly the laboratory’s first approach to answering such questions. However, additional explanations need to be explored when routine tools don’t provide answers. The presence of macroenzymes is one such possibility.
Macroenzymes are serum enzymes found in a complex with high molecular mass components. Macro-forms of many commonly measured enzymes have been identified, including alkaline phosphatase (ALP), amylase, AST, creatine kinase (CK), γ-glutamyl transferase (GGT), lactate dehydrogenase (LDH), and lipase. They may form by binding to serum immunoglobulin (Ig) (referred to as “type 1” macroenzymes) or due to self-polymerization or binding of non-Ig components (“type 2” macroenzymes). CK is the macroenzyme best known for its ability to self-polymerize. Non-Ig components include lipoproteins (reported to bind ALP and GGT) and drug formulations (binding to amylase has been reported). This review will focus on type 1 macroenzymes. Beyond enzymes, hormones and other proteins have also been identified within complexes; e.g., prolactin, TSH, and troponin. The term "macromolecules" will be used to refer to all of these Ig-complexes.
Introducing Macromolecules
Macromolecules are believed to arise from a traditional antigen-antibody interaction between a specific autoantibody and enzyme or hormone. Supporting this theory, an examination of the molecular mass of macroenzyme complexes reveals these interactions occur at the antigen-binding site of the Ig and involve two enzymes bound to a single antibody (1). The presence of Ig and/or the subsequent increase in molecular weight of the complex prolongs the overall half-life of the molecule by impairing its ability to be cleared from circulation or inactivated. These complications result in the elevated enzyme activity or hormone concentrations that laboratories commonly find when these analytes are measured; however, normal concentrations may also be observed (2).
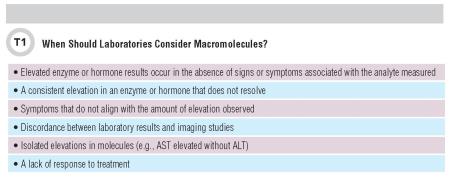
As Davidson and Watson have noted, macroenzymes are “indistinguishable from normal enzymes during routine assay” (3). Ig presence may also affect a hormone’s ability to bind to its functional receptors, thereby altering traditional biological feedback mechanisms. Although elevated values are usually observed, researchers believe that macroenzymes and macrohormones do not exert full biological activity. Typically, they are functionally inert and therefore do not pose an immediate medical concern to patients. Table 1 lists clinical scenarios in which macromolecules should be considered.
Although we have a basic understanding of macromolecules, many mysteries remain. For example, the cause of autoantibody formation against functioning enzyme or hormone has not been fully elucidated. Binding to IgG and IgA (rarely IgM), both monoclonal and polyclonal, have been reported, with some enzymes preferentially binding to one or the other (1).
The association between macromolecule formation and patient disease is also not entirely clear. One case review reported that most patients with macro ALP had autoimmune disease or other autoantibodies (4). However, no obvious or causative association has been defined. At this time, the presumption is that a patient’s disease status is not associated with the presence of macromolecules because these individuals generally are entirely healthy, and in most, no positive correlation with disease has been identified. For these reasons, the presence of macroenzymes and macrohormones is traditionally considered a benign finding.
While prevalence statistics vary considerably and depend upon the analyte and the population studied, macroamylase and macroprolactin are found at higher frequency than other macromolecules. Macroamylase was the first macroenzyme to be identified, more than 50 years ago (5), with its reported prevalence varying between 0.1%-3.5% (6, 7). Macroprolactin prevalence has been reported to be as high as 3%-4% in the general population and may be present in almost 35% of patients presenting with hyperprolactinemia (8). Overall, most macromolecules have been reported more frequently in older individuals, with the exception of macro AST, which is more prevalent in pediatric populations.
Paradoxically, while macromolecules are not considered to be a clinical concern, their effect on analyte measurements may greatly confound the diagnostic process. These benign elevations may prolong diagnostic investigation, prompt inappropriate therapeutic intervention, or simply lead to an erroneous diagnosis. Unfortunately, invasive and costly procedures may result from measuring a biologically inactive form of the enzyme or hormone. In addition, clinicians’ concerns over elevated values may halt the use of essential medications or enrollment in beneficial clinical trials.
To avoid these problems, laboratorians and clinicians absolutely must properly identify these anomalies. Additionally, once a laboratory identifies a macroenzyme or macrohormone, it also should document this anomaly within the patient’s medical record to avoid future confusion or unnecessary investigation, as many reports indicate macromolecules may persist for long periods of time (1). Macro-AST persistence in children has been reported; however, it has also been documented to resolve over time. This finding further emphasizes our lack of understanding of the mechanisms involved in macromolecule formation.
Laboratory Measurement of Macroenzymes: One Size Does Not Fit All
Knowing the importance of detecting a macromolecule to prevent misdiagnosis, laboratorians and clinicians should understand how they are detected. Several detection methods exist, including polyethylene glycol (PEG) precipitation, ultrafiltration (UF), electrophoresis, and gel filtration chromatography (GFC). Although each approach is different, all attempt to separate Ig-bound enzyme or hormone from the unbound, or monomeric, form. Binding to Ig alters the overall size and solubility of the macro-complex, therefore laboratories have used both parameters to measure these analytes. While these are relatively basic principles, laboratorians must be aware of the specifics and limitations of each method.
PEG and UF
PEG precipitation and UF are the most accessible and straightforward of the ways to detect macromolecules. While they are based on different principles, both involve a sample treatment step to separate Ig-bound from monomeric molecules prior to measuring the hormone or enzyme in question. With both PEG precipitation and UF, laboratories must measure the enzyme activity or hormone concentration prior to treating the sample, then calculate a percent recovery or percent activity post-treatment.
UF physically removes Ig-complexes by size. Centrifugation is performed using a filter with a pore size (commonly 100 kDa) that allows for removal of large molecular mass components (Ig-complexes) while still letting the monomeric enzyme or hormone to pass through. PEG precipitation techniques involve adding equal parts of serum to PEG and allowing the two to react. PEG effectively absorbs any available solvent, subsequently increasing the concentration of any remaining proteins. The protein concentration increases until its natural solubility point is exceeded, after which protein precipitation occurs (9).
In serum, this predominantly causes the less-soluble Igs and any Ig complexes to precipitate, albeit nonspecifically. Additionally, it has been reported that IgG preferentially precipitates while IgA partially precipitates (3, 9). This would affect the final recovery of macromolecules commonly found to bind IgA over IgG, such as macroamylase and macro LDH (1). Supporting this theory, we previously reported finding UF methods to be superior to PEG precipitation in detecting these macroenzymes (10, 11). Similarly, many macromolecules have been shown to bind either Ig class, so final concentrations of these analytes may be affected when binding to IgA occurs.
The possibility of falsely precipitating monomeric analytes along with Ig-complexed analytes also limits the overall specificity of the PEG reaction. The extent to which this occurs varies based upon the analyte of interest and again highlights differences between methods as well as their utility for individual analytes. A previous report (12) and a recent case study of a patient with hypergammaglobulinemia (13) indicated false positive macromolecule detection may occur using PEG precipitation in the presence of excess Ig. In these cases, monomeric enzyme was presumed to have been removed concurrently with Ig. UF may therefore be a preferred method in these circumstances because it separates complexes by size alone, and the presence of excess Ig has not been found to affect the final measurement.
Electrophoresis and GFC
Electrophoresis and GFC methods separate Ig-complexes by size and, in the case of electrophoresis, electrical charge. As with UF, the binding of large proteins such as Ig allows for differentiation of bound and monomeric analytes by size. Electrophoresis and GFC methods both require more intensive optimization and instrumentation than PEG precipitation and UF. The choice of gel matrices, buffers, column size, stains, and other laboratory conditions can affect the movement and eventual detection of macromolecules. Electrophoresis and GFC are also more time-intensive and are therefore often considered reference methods and not employed routinely in clinical laboratories. Laboratorians may identify macromolecules by different electrophoretic migration patterns and mobility, enzyme bands, or band intensity. Using GFC, macromolecules will elute prior to free analytes due to their increased mass.
Temperature Activity
Another intriguing characteristic of macromolecules that may aid in their diagnosis is their activity at different temperatures. Laboratories have used thermal stability studies in some cases as a confirmatory technique to identify macroenzymes. Macro CK has been reported to be thermostable, while monomeric CK isoenzymes are thermolabile. However, the sensitivity of heat inactivation is limited at low serum enzyme activities.
AST also has been subjected to temperature sensitivity studies. In 2003, Davidson and Watson observed the gradual decline in total AST enzyme activity in a single case of macro AST (3). Over 90% of the original AST activity was lost when both serum and plasma were stored at 4°C for a period of 6 days. The authors hypothesized that precipitation of Ig-complexes over time caused the declining results. This was also observed in another series of eight patients with more than 50% decline in enzyme activities after 5 or 6 days at 2-8°C (14).
Our own unpublished studies have confirmed this decline in two patients presumed to have macro AST. A third patient tested positive for macro AST by both PEG precipitation and UF, with confirmation by GFC; however, AST enzyme activity remained stable after 6 days storage at 4°C. This highlights an additional complexity of macromolecules that must be appreciated: they are not always predictable, nor consistent.
Cases of Multiple Macromolecules
Isolated elevations in an enzyme or hormone, particularly those unrelated to a patient’s clinical status, may indicate the presence of a macromolecule. Does this mean that macromolecules should be considered only when laboratorians observe elevations in a single enzyme? No. Although rare, there have been documented cases of multiple macromolecules detected concurrently within an individual. Interestingly, when multiple macroenzymes have been detected, they typically are unrelated—for example, CK and LDH or CK, amylase, and AST. The exception is the pancreatic enzymes amylase and lipase, which have been detected together (15).
Many cases are also unique in that the patients have comorbid diseases that may or may not be related to the finding of multiple macroenzymes. For instance, the multiple macroenzymes reported in an HIV-positive individual could be related to the patient’s hypergammaglobulinemic state (13). In this particular case, excess Ig also affected the laboratory method used for macroenzyme diagnosis, with PEG precipitation suggesting the presence of six macroenzymes from the seven tested and UF revealing three. In such rare circumstances, results may depend on the method the laboratory uses, so the patient's original clinical presentation should always be considered.
Implementing Testing
Practical implementation of screening or confirmatory tests such as those outlined in this article requires reference intervals, or diagnostics cutoffs, that can be used in routine practice. Laboratories should develop method-specific reference intervals, particularly for PEG precipitation, as PEG itself may interfere with some immunoassays (9). Establishing positive cutoffs can be challenging because it requires confirmed cases of Ig-complexed enzymes and hormones, and the overall prevalence of macromolecules is quite rare. However, the first step involves understanding the distribution of values in a healthy population for the analyte in question. This data can be used to compare further results, as with traditional reference interval analysis.
Unfortunately, very little published data is available from healthy populations. One study established reference intervals using PEG precipitation in a small number of hospitalized patients (3). Our group has published non-parametric reference intervals for eight different macroenzymes using both PEG precipitation and UF (10, 11). These studies also demonstrated that macroenzymes perform differently depending on the method used, further emphasizing the need for method- and analyte-specific reference intervals.
Conclusion
Macromolecules are unique, relatively rare, and perplexing in their ability to cause an elevated result that has no true biological ramifications. They can cause concern for medical professionals and confusion in clinical laboratories, yet are primarily benign. Understanding and appreciating this phenomenon is essential to avoiding further, unnecessary intervention.
References
- Remaley AT, Wilding P. Macroenzymes: biochemical characterization, clinical significance, and laboratory detection. Clin Chem 1989;35:2261-70.
- Fridhandler L, Berk JE. Macroamylasemia [Review]. Adv Clin Chem 1978;31:267-85.
- Davidson DF, Watson DJ. Macroenzyme detection by polyethylene glycol precipitation. Ann Clin Biochem 2003;40:514-20.
- Crofton PM, Kilpatrick DC, Leitch AG. Complexes in serum between alkaline phosphatase and immunoglobulin G: immunological and clinical aspects. Clin Chim Acta 1981;111:257-65.
- Wilding P, Cooke WT, Nicholson GI. Globulin bound amylase: a cause of persistently elevated levels in serum. Ann Intern Med 1964;60:1053-9.
- Sturk A, Sanders GT. Macro enzymes: prevalence, composition, detection and clinical relevance. Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie 1990;28:65-81.
- Tozawa T. Enzyme-linked immunoglobulins and their clinical significance. Electrophoresis 1989;10:640-4.
- Shimatsu A, Hattori N. Macroprolactinemia: diagnostic, clinical, and pathogenic significance. Clin Dev Immunol 2012;2012:167132.
- Fahie-Wilson M, Halsall D. Polyethylene glycol precipitation: proceed with care. Ann Clin Biochem 2008;45:233-5.
- Wyness SP, Hunsaker JJ, La'ulu SL, Rao LV, Roberts WL. Detection of macro-creatine kinase and macroamylase by polyethylene glycol precipitation and ultrafiltration methods. Clin Chim Acta 2011;412:2052-7.
- Wyness SP, Hunsaker JJ, La'ulu SL, Roberts WL. Reference intervals for six enzymes after polyethylene glycol precipitation and ultrafiltration. Clin Chim Acta 2011;412:1161-2.
- Ram S, Harris B, Fernando JJ, Gama R, Fahie-Wilson M. False-positive polyethylene glycol precipitation tests for macroprolactin due to increased serum globulins. Ann Clin Biochem 2008;45:256-9.
- Wyness SP, Yee MA, La'ulu SL, Tosiello L, Straseski JA. Multiple macroenzymes in a patient with AIDS: diagnosis using ultrafiltration. Am J Clin Pathol 2014;142:266-8.
- Beser OF, Lacinel S, Gulcu D, Kutlu T, Cullu Cokugras F, Erkan T. An easy method for diagnosing macro-aspartate aminotransferase: a case series. The Turkish journal of gastroenterology : the official journal of Turkish Society of Gastroenterology 2014;25:568-70.15. Goto H, Wakui H, Komatsuda A, Imai H, Miura AB, Fujita K. Simultaneous macroamylasemia and macrolipasemia in a patient with systemic lupus erythematosus in remission. Intern Med 2000;39:1115-8.
Joely A. Straseski, PhD, MT(ASCP), DABCC, FACB, is an associate professor at the University of Utah School of Medicine. She is the medical director of endocrinology and co-director of the automated core laboratory at ARUP Laboratories in Salt Lake City. +EMAIL: [email protected]
Sara P. Wyness is a research and development scientist at ARUP Laboratories in Salt Lake City. +EMAIL: [email protected]