If we had to summarize in one phrase our article, “Urine Drug Testing for Monitoring Compliance with Prescribed Opioids” in the August issue of CLN Focus on Mass Spectrometry, it might be “high variance.” Responses from the four pain management laboratory directors we interviewed showed variance in workload, variance in the drugs included in testing menus, variance in cutoffs used to determine positive/negative results, variance in the choice to measure total versus free drug (hydrolysis or no hydrolysis of glucuronidated/sulfated metabolites to free drug), and variance in reporting conventions.
It is then no surprise that our follow-up survey of CLN readers—whom we invited to participate in August through CLN’s Facebook and Twitter accounts and via AACC Artery—found similar divergence in practice. The survey results reinforce what many laboratory medicine professionals have been observing lately, which has given them serious cause for concern.
In total, 97 readers took our 24-question survey, with 38% to 91% responding to each question. Our respondents might have all come from different labs, or there could have been more than one response from the same labs, as we did not have controls to prevent multiple people working at one laboratory from answering the survey individually and being counted as separate laboratories. We will provide a more detailed description of the survey results in a subsequent CLN issue. Many participants provided free text responses, and this data will take more time to analyze.
Respondents work primarily in small- and medium-sized laboratories, with 43% testing <50 samples per day and 35% testing 50-500 samples per day. Nearly three-quarters reported having five or fewer mass spectrometry systems. Physician office laboratorians most likely were underrepresented in our survey, as fewer of these individuals read CLN in comparison to those working in hospital or commercial reference laboratories and we saw correspondingly fewer responses from the former.
Our survey showed that while automated sample preparation and data analysis appear to be increasing, many labs still perform these activities completely manually—46% do all manual sample preparation and manual data analysis. In contrast, fewer than 20% reported having totally automated sample preparation and data analysis.
Most survey participants perform a variety of testing. Slightly less than half (46%) indicated that pain management testing for opoids comprises the majority of their workload, meaning that at least 50% of their opioid-related testing is for monitoring pain management patients as opposed to monitoring substance abuse treatment, managing patients in the emergency department or for other purposes. This is a small sample, but these responses support the perception that pain management testing occurs in a wide variety of testing venues—not just those dedicated to the subspecialty.
In near-equal thirds, respondents reported serving local, regional, or national client bases, and 73% indicated that they have College of American Pathologists accreditation. The results also revealed that 77% of participants offer a pain management multi-drug class panel, with two-thirds offering >20 analytes in the panel. As we expected, respondents’ positive/negative cutoffs varied widely; results for morphine are shown in Figure 1. We will provide the full set of cutoff data in our final survey report.
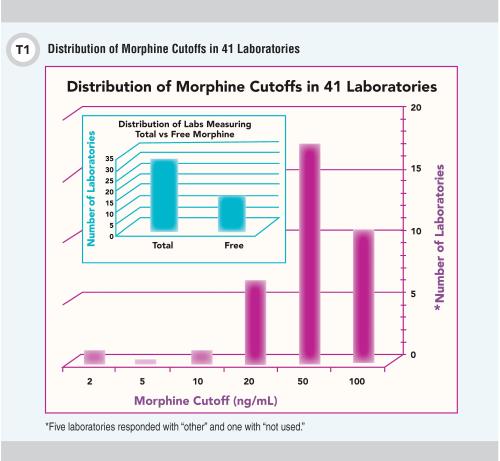
To serve patients and clinicians most effectively the laboratory medicine community clearly needs to develop consistent standards of practice for pain management drug testing. The release for public comment of the AACC Academy (formerly known as NACB) Laboratory Medicine Practice Guideline: Using Clinical Laboratory Tests to Monitor Drug Therapy in Pain Management Patients is an admirable step towards this goal (1).
However, the AACC Academy guideline found insufficient evidence to recommend specific cutoff concentrations or whether hydrolyzing glucuronide/sulfate metabolites is necessary. The guideline does state by consensus expert opinion that use of lower limit-of-detection cutoff concentrations can be more effective to detect use.
This uncertainty and our survey results suggest that high variance is also likely in the drug/metabolite concentrations or positive/negative status that would be reported for the same patient sample by different pain management testing laboratories. External quality assurance/proficiency testing (PT) surveys are expected to address this issue, but PT samples for urine drug testing do not always include glucuronide/sulfate metabolites, evaluate quantitative accuracy, or require low detection limits in the presence of high concentrations of similar compounds. Lorin Bachmann, PhD, in this issue emphasizes that simply performing a test using an LC-MS/MS method does not by default equate with acceptable accuracy/trueness. She describes the considerable effort needed to improve between-laboratory agreement for 25-OH Vitamin D and testosterone (2). Unfortunately, certified reference procedures (CRP) and materials (CRM) are not available from NIST to assess analytical accuracy for pain management urine drug testing. It seems that evaluating, and if necessary improving, between-laboratory agreement using commutable samples that include analytes in the form and concentrations typical of pain management patient samples is also needed.
In the past, reimbursement pressures and regulatory controls forced change when such broad discrepancies existed. That scenario is poised to be played out again unless the clinical laboratory community voluntarily acts to agree on practice standards and validate analytical accuracy. Urine drug testing was on the list of the top 25 clinical laboratory tests reimbursed by Medicare in 2014 (2). It will be up to us as a profession to tackle a problem with testing that costs our fellow taxpayers millions of dollars every year but on which we do not agree about the most basic principles of analysis and reporting.
References
1. Kirkwood J. New guidance on pain management testing. Clinical Laboratory News 2016;42(7):34-38.
2. Medicare payments for clinical laboratory tests in 2014: Baseline data (OEI-09-15-00210). Department of Health and Human Services, Office of the Inspector General, HHS OIG Data Brief, September 2015.
Shannon Haymond, PhD, is the director of clinical chemistry and mass spectrometry at Ann & Robert H. Lurie Children’s Hospital of Chicago and assistant professor of pathology at Northwestern University Feinberg School of Medicine. +Email: [email protected]
Judith Stone, PhD, is the senior clinical laboratory scientist specialist at the University of San Diego toxicology laboratory in the Center for Advanced Laboratory Medicine.+Email: [email protected]
CLN's Focus on Mass Spectrometry is sponsored by Waters Corporation.
