After two decades without much change in HIV laboratory testing, suddenly there are many testing options and more than a few questions from laboratorians and physicians alike. The new testing strategy capitalizes on recent advances in technology and new Food and Drug Administration (FDA)-approved assays in the U.S.
The Centers for Disease Control and Prevention (CDC) published the most recent recommendations for HIV laboratory testing, including a new algorithm, in 2014. This algorithm calls for screening with a laboratory-based antigen/antibody combination or fourth-generation assay, with follow-up of any reactive screening results using an HIV-1/HIV-2 differentiation assay to confirm HIV-1 and/or HIV-2 antibodies. Specimens with a reactive screen and negative or indeterminate results by the differential assay should be followed up with nucleic acid testing (NAT) to identify acute HIV infection (AHIV). This algorithm improves detection of both HIV-2 and acute HIV-1 infections.
When the algorithm was first proposed there were limited options for each step in the algorithm, but today we have several antigen/antibody screening assays, including a rapid test, two very different options for HIV-1/HIV-2 differentiation assays, as well as laboratories using viral load assays for NAT. Navigating the ever-changing sea of options for HIV testing is becoming more challenging.
Options for Antigen/Antibody Combination Assays
Laboratory-Based Antigen/Antibody Assays
As the new algorithm has gained traction, the menu of laboratory-based, FDA-approved antigen/antibody combination assays also has increased and now stands at four: the ADVIA Centaur HIV Ag/Ab Combo assay (Siemens); ARCHITECT HIV Ag/Ab Combo assay (Abbott Diagnostics); and the BioPLex 2200 HIV Ag-Ab and GS HIV Combo Ag/Ab EIA assays (Bio-Rad Laboratories). The BioPlex assay not only detects but also differentiates between HIV-1 antibodies, HIV-2 antibodies, and HIV-1 p24 antigen, and sometimes is referred to as a fifth-generation HIV assay.
Antigen/antibody (ag/ab) combination assays not only detect established infection in those who have seroconverted but also identify HIV infection prior to seroconversion by detecting p24 antigen. Many studies have demonstrated the ability of fourth-generation assays to improve detection of acute HIV cases, particularly in high risk populations (1, 2). Fourth-generation assays also detect acute HIV infections 5–7 days earlier, on average, compared to third-generation, antibody-only assays. Early detection of HIV infection in individuals who have not seroconverted reduces disease transmission and directly benefits patients, who can receive prompt therapy, leading to improved health outcomes.
Data on performance characteristics for HIV antigen/antibody assays are largely derived from published studies examining the performance of each platform individually (3, 4). The CDC 2014 HIV laboratory testing recommendations include an extensive table of studies that CDC reviewed for the assays’ performance characteristics in guiding the agency’s final recommendations (5).
Only one study compared two antigen/antibody combination assays (Architect and GS) with two third-generation assays (Ortho Anti-HIV-1+2 EIA and ADVIA Centaur Enhanced HIV-1/O/2). However, not a single independent field study conducted in the U.S. has directly compared the three available antigen/antibody laboratory-based methods that have similar chemistry: ADVIA Centaur HIV Ag/Ab Combo; ARCHTIECT HIV Ag/Ab Combo; and GS HIV Combo Ag/Ab EIA (6). Limitations to performing these types of studies include access to multiple platforms (FDA only recently approved the ADVIA Centaur HIV Ag/Ab Combo assay), the large volume of samples needed to reveal small differences between platforms, and expensive reagents needed for parallel and resolution testing.
Depending on the study, the following parameters are usually evaluated: analytical sensitivity using a World Health Organization p24 Ag standard; the ability to detect acute HIV infection using seroconversion panels; the ability to detect various HIV groups and subtypes using known samples; and assessing specificity in low risk populations, such as blood donors. Studies typically evaluate one assay for all parameters or compare two methods at a time for many of the parameters. Data from several investigators have shown that although there are differences in all of these parameters among fourth-generation assays, in general, the differences are not statistically significant (7–9).
A longstanding concern about fourth-generation assays has been specificity and the positive predictive value of the antigen/antibody assays in low prevalence populations. Data suggest that there is not a significant increase in the number of false positive results observed when an antigen/antibody combination assay
is used in a low incidence population (10). One study investigating the Architect platform determined that there is a positive correlation between the signal-to-cutoff ratio and positive predictive value (11). However, it is important to remember that these all are screening assays and even when specificity is very high, >99.5% for example, the positive predictive value in low risk populations may be <50%. This reinforces the need for supplemental testing.
Another practical parameter to consider is the sample type. First, performance characteristics such as sensitivity and specificity can vary slightly depending on the sample type, even for the same kit. In addition, interference and incorrect results obtained with serum versus plasma samples for the same antigen/antibody combination assay also have been reported (12). Furthermore, not all fourth-generation combination assays on the market are approved for both plasma and serum samples. Although both of the HIV-1/HIV-2 differentiation assays are approved for both sample types, some HIV viral load assays that may be used by laboratories to detect HIV-1 RNA only allow for plasma samples.
Rapid Antigen/Antibody Combination Test
In 2013 FDA approved the first antigen/antibody rapid test, the Alere Determine HIV-1/2 Ag/Ab Combo. The Determine Ag/Ab assay simultaneously detects in serum, plasma, and whole blood specimens HIV-1 p24 antigen and antibodies to both HIV-1 and HIV-2. It not only detects both anti-HIV antibodies and p24 antigen but also distinguishes between antibody- and antigen-positive HIV samples. Therefore, a specimen can be classified as antibody-positive, antigen-positive, antigen- and antibody-positive, or antigen- and antibody-negative. Initial data suggested that the rapid ag/ab combination assay was not as sensitive for detecting acute HIV infections as laboratory based antigen/antibody combo assays. The assay was reformulated in 2011 and at least one study has demonstrated improved sensitivity for detecting acute HIV infections (13).
The availability of this first ag/ab combo rapid test generated a lot of excitement because many were hoping to use this test as the first step in the new algorithm, substituting it for a laboratory-based fourth-generation assay. However, the 2014 CDC guidelines cited insufficient evidence in support of using the rapid antigen/antibody combination test as the initial step in the algorithm. As a result, all reactive rapid test results must be followed-up with a laboratory-based ag/ab combination assay (5).
HIV-1/HIV-2 Differentiation Assay: The Switch
When the CDC recommendations were published there was only one FDA-approved HIV-1/HIV-2 differentiation assay, the Multispot HIV-1/HIV-2 Rapid Test (Bio-Rad Laboratories). As of October 2014, laboratories also may use the Bio-Rad Geenius HIV 1/2 Supplemental Assay. The Multispot has become synonymous with an HIV-1/HIV-2 differentiation assay, and is a flow-through rapid test, with interpretation based on presence of reaction at four spots (Figure 1). In addition to the procedural control spot there are two spots for detecting anti-HIV-1 antibodies—both corresponding to gp41—and one for anti-HIV-2 antibodies, gp36. Interpreting results is straightforward: HIV-1 or HIV-2 antibody positive, HIV-1/HIV-2 undifferentiated, HIV-1/HIV-2 negative, and rarely, HIV-1 indeterminate.
Bio-Rad originally developed the Multispot as a screening test. When CDC recommended an HIV-1/HIV-2 differentiation assay as the second step in the algorithm, the Multispot, which was the only assay with this capability, was not FDA-cleared for use as a supplemental assay. Bio-Rad subsequently sought FDA approval for supplemental use and it was approved with one important change that affected interpretation: if the Multispot is used as a supplemental assay, a positive interpretation for HIV-1 antibodies requires that there be a reaction at both HIV-1 spots, not just one. If only one HIV-1 spot is reactive then the result is “HIV-1 indeterminate” (Figure 1).
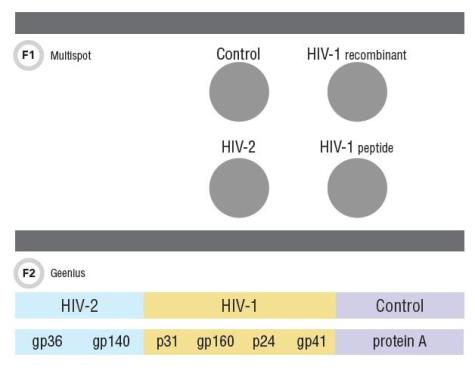
This change was based on data from several studies suggesting that reaction at both HIV-1 spots increased the specificity of the assay, which is critical for a confirmatory assay. The different interpretation for screening versus confirming a repeatedly reactive screen presented a challenge for laboratories. But the rapid test format works well in small primary care institutions for confirming HIV infections and offers significantly improved turnaround time, as most infections are established and would be positive at this second step of the algorithm. However, the Multispot will no longer be available as of the end of 2016.
The alternative HIV-1/HIV-2 differentiation assay, the Geenius, also is a single-use test FDA approved for confirming reactive HIV screen results and differentiating between HIV-1 and HIV-2. The test uses either recombinant or synthetic peptides corresponding to four HIV-1 antigens—gp160, gp41, p31, and p24—and two corresponding to HIV-2 antigens, gp140 and gp36 (Figure 2). The results can be read within 30 minutes and interpreted using an automated cassette reader and a proprietary algorithm. The automated interpretation feature eliminates the need for review by trained personnel and reduces inter-observer subjectivity.
There are a total of six possible interpretations based on the pattern observed. In addition to HIV-negative, HIV-1-positive, HIV-2-positive, HIV-positive, and untypable, other possible results include HIV-2 indeterminate and HIV-2-positive with HIV-1 cross-reactivity. The cassette system allows for a bar code label on each specimen, improving traceability and reducing sample mix-up errors. Additionally, because software is necessary for interpretation, the results are digitally captured and automatically recorded, unlike Multispot results that can only be interpreted a few hours after performing the test. The software allows laboratories to store an electronic copy of the visual record and associated interpretation.
Beyond the significant, practical improvements in sample traceability and result documentation, the important question is how the Geenius performs as a confirmatory HIV-1/HIV-2 antibody differentiation assay. According to a recent study that compared the CE-approved version of the Geenius and the Multispot, the two assays were comparable in performance and had low bias and a high kappa value (0.96), indicating good agreement.
Both the Geenius and the Multispot also accurately classified rare, HIV-1 non-B genotypes and low titer samples. Sensitivity was 100% for both assays. Specificity values were 99.1% for Multispot and 96.3% for Geenius in the Canadian population investigated in the study, which has a very low HIV prevalence (0.3%). The authors also noted that they determined specificity using a single test approach and not using either the Multispot or the Geenius as part of an algorithm. This might have eliminated some false positive results because they might have been called negative using an antigen/antibody screen test. Both assays had similar ability to differentiate HIV-1 from samples—100% for Multispot and 99.2% for Geenius. The differentiation rate for HIV-2 samples were 100% and 99.2% for Multispot and Geenius, respectively. The differentiation rate for HIV-2 samples was 98.1% for both assays (14). The differentiation rate differences between the two assays were not statistically significant, for either HIV-1 or HIV-2.
A significant, practical advantage is that Geenius, unlike Multispot, does not require dilutions for final differentiation between HIV-1 and HIV-2 antibody positive samples. Another benefit to Geenius is that it is approved for use in pediatric patients 2 years of age or older.
One drawback to Geenius is that it requires laboratories to purchase the automated reader. Although the system is relatively inexpensive, opting for the Geenius system and associated service costs is more involved than choosing an assay that is limited only by the cost per test like Multispot. The lack of access to Multispot may cause small primary care institutions to forgo offering HIV-1/HIV-2 supplemental testing in house and revert back to sending confirmatory testing to a reference laboratory. Such a change has the potential to have a negative impact on patient care, as one of the benefits of using a rapid test to confirm HIV infection was providing quick, reliable results to the patient.
The Future
What is on the horizon? The BioPLex 2200 assay became available as of August 2015. This assay can differentiate between antigen, HIV-1 antibody, and HIV-2 antibody positive samples. Because this technology essentially determines in one pass whether the patient has acute HIV-1, established HIV-1, or HIV-2 infection, it provides a significant advantage. What effect this type of assay might have on future guidelines remains to be seen, as results may dictate very different follow-up protocols. For example, if the patient is positive for HIV-1 p24 antigen will labs be able to proceed directly to NAT? Stay tuned as HIV laboratory testing algorithms may soon change again.
References
- Goodhue T, Kazianis A, Werner BG, et al. 4th generation HIV screening in Massachusetts: A partnership between laboratory and program. J Clin Virol 2013;58 Suppl 1:e13–8.
- Manlutac AL, Giesick JS, McVay PA. Identification of early HIV infections using the fourth generation Abbott Architect HIV Ag/Ab Combo Chemiluminescent Microparticle Immunoassay (CIA) in San Diego county. J Clin Virol 2013;58 Suppl 1:e44–7.
- Bentsen C, McLaughlin L, Mitchell E, et al. Performance evaluation of the Bio-Rad Laboratories gs HIV Combo Ag/Ab EIA, a 4th generation HIV assay for the simultaneous detection of HIV p24 antigen and antibodies to HIV-1 (groups M and O) and HIV-2 in human serum or plasma. J Clin Virol 2011;52 Suppl 1:S57–61.
- Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott Architect HIV Ag/Ab Combo Assay. J Clin Virol 2011;52 Suppl 1:S51–5.
- Branson BM, Owen MS, Wesolowski LG, et al. Laboratory testing for the diagnosis of HIV infection: Updated recommendations. 2014.
- Mitchell EO, Stewart G, Bajzik O, et al. Performance comparison of the 4th generation BioRad Laboratories GS HIV Combo Ag/Ab EIA on the Evolis automated system versus Abbott Architect HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 Enhanced on Advia Centaur. J Clin Virol 2013;58 Suppl 1:e79–84.
- Vallefuoco L, Aden Abdi F, Sorrentino R, et al. Evaluation of the Siemens HIV antigen-antibody immunoassay. Intervirology 2014;57:106–11.
- Miedouge M, Greze M, Bailly A, et al. Analytical sensitivity of four HIV combined antigen/antibody assays using the p24 WHO standard. J Clin Virol 2011;50:57–60.
- Pumarola T, Freeman J, Saxton E, et al. Performance evaluation of the Advia Centaur HIV Ag/Ab Combo assay. J Virol Methods 2010;170:16–20.
- Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol 2013;58 Suppl 1:e76–8.
- Jensen TO, Robertson P, Whybin R, et al. A signal-to-cutoff ratio in the Abbott Architect HIV ag/ab combo assay that predicts subsequent confirmation of HIV-1 infection in a low-prevalence setting. J Clin Microbiol 2015;53:1709–11.
- Akl P, Blick KE. A case of false-positive test results in a pregnant woman of unknown HIV status at delivery. Lab Med 2014;45:259–63.
- Masciotra S, Luo W, Youngpairoj AS, et al. Performance of the Alere Determine HIV-1/2 Ag/Ab combo rapid test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol 2013;58 Suppl 1:e54–8.
- Malloch L, Kadivar K, Putz J, et al. Comparative evaluation of the Bio-Rad Geenius HIV-1/2 confirmatory assay and the Bio-Rad Multispot HIV-1/2 rapid test as an alternative differentiation assay for clsi m53 algorithm-i. J Clin Virol 2013;58 Suppl 1:e85–91.
Patricia Slev, PhD, DABCC, is an associate professor of pathology at University of Utah School of Medicine and medical director of the serologic hepatitis and retrovirus laboratory and immunology core laboratory at ARUP Laboratories in Salt Lake City. +Email: [email protected]