[Download pdf] 
Authors
Christina C. Pierre
Department of Pathology and Laboratory Medicine
Penn Medicine Lancaster General Hospital
Lancaster, PA, United States
Department of Pathology and Laboratory Medicine
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA, United States
Mark A. Marzinke
Departments of Pathology and Medicine
Johns Hopkins University
School of Medicine
Baltimore, MD, United States
Sofia B. Ahmed
Department of Medicine
Cumming School of Medicine
University of Calgary
Calgary, AB, Canada
Population Health Research Institute
Hamilton, ON, Canada
David Collister
Division of Nephrology
University of Alberta
Edmonton, AB, Canada
Population Health Research Institute
Hamilton, ON, Canada
Jessica M. Colón-Franco
Department of Laboratory Medicine
Cleveland Clinic
Cleveland, OH, United States
Melanie P. Hoenig
Department of Medicine
Harvard Medical School
Boston, MA, United States
Division of Nephrology and
Department of Medicine
Beth Israel Deaconess Medical Center
Boston, MA, United States
Thomas Lorey
Kaiser Permanante
The Permanante Medical Group
Regional Laboratory
Berkeley, CA, United States
Paul M. Palevsky
Renal-Electrolyte Division
Department of Medicine
University of Pittsburgh
Pittsburgh, PA, United States
Kidney Medicine Program and Kidney Medicine Section
Veterans Affairs Pittsburgh Healthcare System
Pittsburgh, PA, United States
The National Kidney Foundation, Inc.
New York, NY, United States
Octavia Peck Palmer
Departments of Pathology, Critical Care Medicine, and Clinical and Translational Science
University of Pittsburgh
School of Medicine
Pittsburgh, PA, United States
Sylvia E. Rosas
Kidney and Hypertension Unit
Joslin Diabetes Center and Harvard Medical School
Boston, MA, United States
Division of Nephrology, Department of Medicine
Beth Israel Deaconess Medical Center
Boston, MA, United States
The National Kidney Foundation, Inc.
New York, NY, United States
Joseph Vassalotti
Division of Nephrology, Department of Medicine
Icahn School of Medicine at Mount Sinai
New York, NY, United States
The National Kidney Foundation, Inc.
New York, NY, United States
Cameron T. Whitley
Department of Sociology
Western Washington University
Bellingham, WA, United States
Dina N. Greene
Department of Laboratory Medicine and Pathology
University of Washington Medicine Seattle, WA, United States
LetsGetChecked Laboratories
Monrovia, CA, United States
Citation
Pierre CC, Marzinke MA, Ahmed SB, Collister D, Colón-Franco JM, Hoenig MP, et al. AACC/NKF guidance document on improving equity in chronic kidney disease care. [Epub] J Appl Lab Med June 27, 2023, as doi: 10.1093/jalm/jfad022
Introduction
Despite significant progress in disease diagnosis and treatment, racial and ethnic minorities experience lower quality of care and poorer outcomes for several health conditions compared to nonminorities. The federal government has acknowledged and researched these disparities extensively for >3 decades (1). In 2020, the widely publicized and tragic deaths of multiple Black individuals heightened collective calls to examine and mitigate the impacts of systemic racism on racialized minority populations. On the heels of these events, the disproportionate burden of COVID-19 morbidity and mortality experienced by racialized minorities galvanized momentum for change across several institutions, including healthcare (2, 3). A complex interplay of biological and social factors influence racial and ethnic healthcare disparities. These disparities can also be perpetuated and exacerbated by systemic healthcare practices (2–4). One such practice, the use of Black race coefficients in estimated glomerular filtration rate (eGFR) equations, prompted the formation of a joint task force by the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) to reassess the inclusion of race in diagnosing kidney diseases (KDs), risk stratification, and classification. The NKF-ASN Task Force detailed their recommendations and rationale in interim and final reports (4, 5). The recommendations can be summarized as follows:
- All laboratories should immediately implement the CKD-EPI 2021 (Chronic Kidney Disease Epidemiology Collaboration) creatinine-based eGFR (eGFRcr) equation that was developed without the use of the race coefficient for US adults.
- National efforts should be undertaken to facilitate increased, routine, and timely use of cystatin C, in populations at risk for chronic kidney disease (CKD) or who have CKD. The race-neutral CKD-EPI 2012 eGFRcys and CKD-EPI 2021 eGFRcr-cys equations should be adopted to provide more accurate first-line or confirmatory testing, as appropriate for the clinical setting.
- Research on GFR estimation with new endogenous filtration markers and on interventions to eliminate racial and ethnic disparities in KD should be encouraged and funded.
The purpose of this guidance document is to provide a tool to facilitate the implementation of the NKF-ASN Task Force recommendations in clinical laboratories. In addition to discussing practical aspects of implementing the CKD-EPI 2021 eGFRcr equation and cystatin C testing, the document explores CKD risk factors, laboratory tests for diagnosing and managing CKD, and recommendations on appropriate use of cystatin C-based eGFR equations. A framework for understanding the nuances and potential harms of utilizing race as a biological classifier in eGFR is provided and details evidence-based, actionable measures that clinical laboratorians can take to improve equity in kidney health. Race, ethnicity, and gender identity can intersect to impact how individuals receive healthcare (6). Greater attention to GFR reporting and its challenges also highlights the importance of appropriate use of eGFR in transgender and gender-diverse individuals. Therefore, considerations for eGFR reporting in these populations are also discussed.
What Groups are at Risk for Worse Disease Burdens and Outcomes from CKD?
Key summary points:
- Clinical risk factors for KD include diabetes, hypertension, a family history of kidney failure, and cardiovascular disease.
- Racial and ethnic minorities and individuals with low socioeconomic status experience worse kidney health and clinical outcomes.
- Individuals with 2 APOL1 risk alleles have a significantly greater risk of KD; these alleles are most prevalent in individuals of recent West African ancestry.
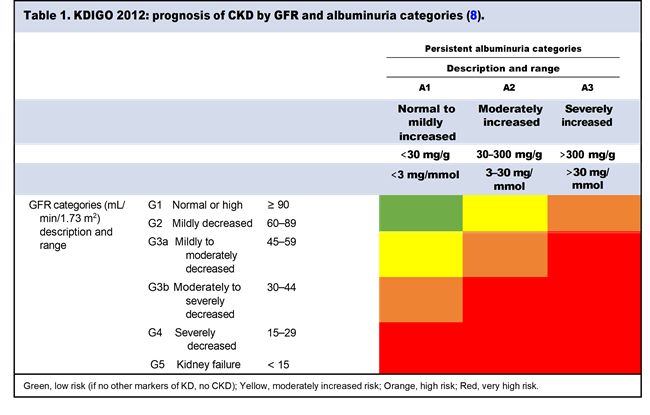
CKD is a heterogeneous group of disorders and remains a global public health concern (7). The Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines define CKD as abnormalities of kidney structure or function, present for >3 months, with implications for health (8). For CKD diagnosis, either one or more markers of kidney damage (including albuminuria) or GFR <60 mL/min/1.73 m2 must be present for >3 months. CKD is classified by identifying the cause of CKD (C), assigning a GFR category (G), and assigning an albuminuria category (A), which is collectively known as “CGA Classification” (8). CKD is classified according to 3 albuminuria and 6 GFR categories as described in Table 1.
In 2021, the Centers for Disease Control and Prevention estimated that 37 million adults in the United States have CKD (7). Complex interactions of social, environmental, and biological factors are associated with CKD. Women exhibit a higher prevalence of CKD (15.4% vs 12.6%) (9), however men have a higher risk of developing kidney failure (64.6% higher) (9–12). CKD is more prevalent in Black non-Hispanic (16.3%) and Black Hispanic (13.6%) adults than White and Asian non-Hispanic and White Hispanic adults (12.7% and 12.9%, respectively) (7, 13). Black individuals are at a 3.8 times higher risk for kidney failure and are 1.4 times less likely to receive a kidney transplant compared to White individuals. Hispanic individuals are at a 2.1 times higher risk for kidney failure and are 1.3 times less likely to receive a kidney transplant compared to White individuals (9). White persons are more likely to be placed on the waitlist for a kidney transplant prior to dialysis initiation, and more likely to receive a living donor kidney transplant (rate of 1.3 per 100 patient-years) while on dialysis as compared to Black (rate of 0.3 per 100 patient-years) and Hispanic individuals (rate of 0.6 per 100 patient-years) (9, 14).
Clinical risk factors for CKD include diabetes mellitus, hypertension, cardiovascular disease, obesity, history of acute kidney injury, older age (≥65 years), suboptimal diet (including high intake of animal protein, and low intake of fruits and vegetables) (7, 15, 16), hereditary kidney disorders (17, 18), and the presence of APOL1 KD risk variants (7, 19). Social determinants of health also contribute to CKD incidence, prevalence, and morbidity. The social deprivation index (SDI) quantifies socioeconomic variation in health outcomes by measuring area level deprivation based on 7 data points (income, education, employment, housing, household characteristics, transportation, and demographics) collected in the American Community Survey (20). A higher SDI indicates a higher level of combined socioeconomic stressors. Black and Hispanic Medicare recipients are over-represented in high SDI neighborhoods (58.6% and 65.1%, respectively) compared to White Medicare recipients (21.5%). Individuals who experience the most deprivation also experience worse kidney health and healthcare compared to those in low SDI neighborhoods, irrespective of race (14). Nevertheless, within SDI cohorts, racial and ethnic disparities in end-stage kidney disease (ESKD) incidence and preemptive kidney transplant remain evident (9).
Genetic variants play a clear role in increasing risk of KD in some Black individuals. Individuals with 2 risk variants for the gene that encodes apolipoprotein L1 (APOL1) are at significantly greater risk for developing many types of severe KD (21, 22). These risk alleles are more prevalent in individuals of recent West African ancestry (21, 22). The presence of 2 risk alleles confers a significantly greater risk of hypertension-attributed ESKD, focal segmental glomerulosclerosis, and HIV associated nephropathy. APOL1 “KD variants” are not 100% penetrant and more research is needed to assess the impact of environmental and psychosocial factors on gene expression in KD.
WHAT TESTS ARE USED TO DIAGNOSE AND MANAGE CHRONIC KIDNEY DISEASE?
Key summary points:
- Patients with risk factors for CKD should be clinically evaluated and monitored with measurements of creatinine and/or cystatin C to determine eGFR, and with uACR (urine albumin–creatinine ratio) to assess albuminuria.
- The Kidney Profile, which combines eGFR and uACR together under one heading on the laboratory requisition form or electronic health record order, can simplify test ordering for detection, diagnosis, and monitoring progression of CKD.
Clinical laboratory tests used to diagnose and manage CKD include creatinine, cystatin C, GFR (measured or estimated), and uACR. Table 2 includes a brief summary of recommendations for measuring and reporting of each test and eGFR equation.
Creatinine
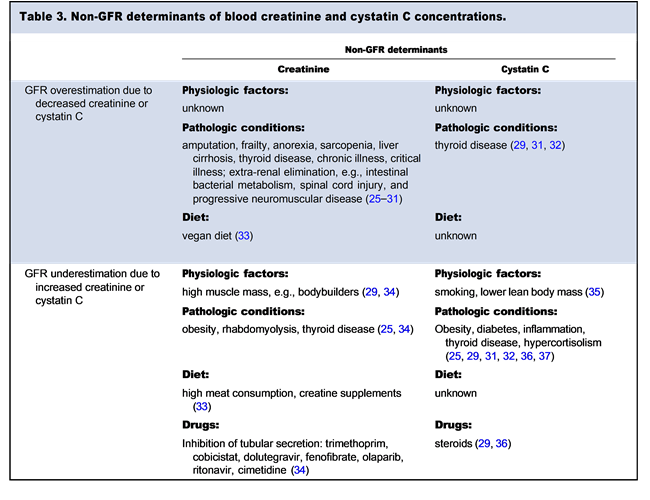
Creatinine, a catabolic product of muscle metabolism, is measurable in blood and urine. Normally, creatinine is generated at a constant rate and is freely filtered by the glomerulus. In addition, the tubules in normally functioning kidneys secrete 6–8% of blood creatinine. When kidney function decreases, the tubules secrete as much as 30% of blood creatinine (24). Of note, creatinine is an imperfect marker of GFR because several non-GFR determinants can affect systemic creatinine concentrations independently of GFR. Table 3 describes non-GFR determinants of blood creatinine.
Cystatin C
Cystatin C is a 13.3 kDa protease inhibitor that is synthesized in all nucleated cells, freely filtered through the glomerular membrane, and resorbed and catabolized in the proximal tubules (38). Cystatin C has been established as an alternative and adjunct to creatinine in GFR estimation (38-40). Equations that incorporate both markers show superior performance compared to those relying solely on creatinine or cystatin C (39, 40). Furthermore, cystatin C has utility as a marker for acute kidney injury (AKI) in certain settings (41, 42). Cystatin C also has non-GFR determinants, as described in Table 3, and these determinants may be enriched in hospitalized individuals.
Measured and Estimated Glomerular Filtration Rate
Direct evaluation of GFR requires blood or urinary clearance of exogenous analytes that are filtered, but not resorbed or secreted by the kidney. Common agents used to measure GFR directly include inulin, iothalamate, and iohexol. Traditionally, inulin was the gold standard for measured GFR (mGFR), but it is not currently available in the United States. Serial blood samples are collected to determine clearance kinetics of these agents. Urinary clearance of creatinine can be measured with a single blood sample, but requires a timed urine collection, which is inconvenient and subject to errors in completeness of collection. eGFR has predominantly replaced mGFR and measured creatinine clearance in most clinical practice due to practical and cost considerations. GFR estimation equations utilize the concentration of endogenous filtration markers such as creatinine and/or cystatin C in the blood to evaluate GFR together with demographic characteristics as surrogates of the non-GFR determinants. Prior to the introduction of the CKD-EPI 2021 refit equations, the 4-variable Modification of Diet in Renal Disease (4v-MDRD) and 2009 CKD-EPI eGFRcr equations were the most commonly used creatinine-based eGFR equations in the United States. Both equations were derived using blood creatinine in conjunction with age, sex, and a Black race coefficient, resulting in an indexed eGFR that is standardized to a body surface area (BSA) of 1.73 m2 (the average BSA of a 70 kg man). The CKD-EPI 2021 refit equations incorporate blood creatinine (eGFRcr), or creatinine and cystatin C (eGFRcr₋cys), along with age and sex. The CKD-EPI 2021 eGFR equations are detailed in Table 2.

Urine Albumin to Creatinine Ratio
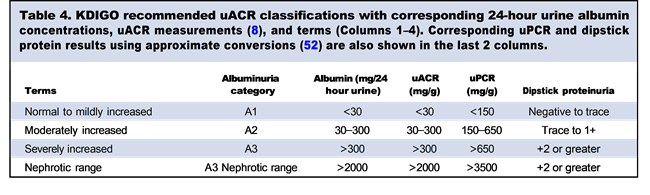
The urine albumin to creatinine ratio(uACR) helps to estimate the amount of albumin excreted in the urine over 24 hours based on assumptions regarding creatinine excretion. uACR is a marker of kidney damage, which may be elevated in the absence of decreased GFR. uACR is used in conjunction with eGFR to detect CKD, classify its severity, and assess risk for CKD progression and complications. Normally, the glomerulus filters only a small quantity of albumin, which is nearly completely resorbed in the proximal tubules via active transport processes. In the setting of high quantities of albumin entering the filtrate due to glomerular disease, or compromised tubular function, active transport mechanisms become saturated, leading to excretion of albumin into the urine (43). Urinary albumin should be normalized to urine creatinine and reported as a ratio (uACR). Normalization to creatinine accounts for variability in dilution and concentration of the urine and overall water balance. uACR may be determined from a random urine sample or a 24-hour collection. Normalization of urine albumin to urine creatinine in a 24-hour urine collection may not be necessary if the value of interest is the albumin excretion rate in mg/min or mg/24 h. uACR results <10 mg/g of creatinine are optimal, 10-30 mg/g is mildly increased, 30-300 mg/g is moderately increased, and >300 mg/g is markedly increased (8, 44). Historically, the term microalbumin was used as a pseudonym for urine albumin or uACR determinations. This term is a misnomer, and current recommendations advocate that the term urine albumin be used to describe the individual measurement, and that uACR be used as the indicator of albuminuria (8).
Urine albumin assays are not standardized, which precludes the application of uniform clinical decision points in the assessment of albuminuria between laboratories that use different assays (45, 46). While most urine albumin assays are relatively precise, with coefficients of variation ranging between 0.5% and 3.8%, assay bias relative to isotope dilution-LC-MS reference assays causes lack of agreement among assays (47). Patients should be screened and monitored using serial urine albumin measurements by the same assay to calculate the uACR. Standardization efforts are underway to enable better agreement between measurements performed at different laboratories (45, 46). It should be emphasized that urine albumin and uACR exhibit large intraindividual biological variation, which can be larger than the differences observed between albumin measurements using assays from different manufacturers (45, 46, 48).
Several commercially available urine albumin assays are limited by their lower limit of quantification (LLOQ), which prevents precise calculation of the uACR when urine albumin is below the LLOQ. In these instances, Miller et al. recommend using the manufacturer-defined LLOQ as the numerator in the uACR calculation; however, this strategy can result in falsely increased rates of uACRs above the clinically important threshold of 30 mg/g (49, 50). As an alternative, the clinical laboratory can validate lower limits of quantification. Changing the LLOQ would render an assay FDA-modified and, as such, a thorough validation study would be required. Greene et al. validated a decreased LLOQ of 3 mg/L for urine albumin, compared to the manufacturer-defined LLOQ of 12 mg/L (50). Serial dilutions were used to assess linearity at the lower LLOQ. Serial measurements of diluent blank and low concentration samples were used to calculate the limit of blank and limit of detection. Imprecision of ≤10% was considered acceptable for a reduced LLOQ. Urine samples with measured albumin concentrations <12 mg/L were compared with an external laboratory's assay with a validated LLOQ of 3 mg/L. This study found that among specimens with urine albumin concentrations of <12 mg/dL, 0.4% (2/499) had uACRs of >30 mg/g, compared to 21.4% specimens when the manufacturer-defined LLOQ was substituted as the numerator in the uACR calculation (50).
uACR in mg/g is preferred to urine protein to creatinine ratios (uPCR), due to its improved clinical specificity and sensitivity (51). Currently, KDIGO guidelines stratify 3 uACR categories, as shown in Table 1. Further, in a recent meta-analysis, efforts were made to correlate uPCR or qualitative urine protein results with uACR results (Table 4) (52). Such correlations may prove useful in their ability to categorize patients by albuminuria classification in instances where only urinary protein measurements are available. uPCR is more variable and less reliable at lower urine protein concentrations due to large differences in the reagents used for measurement.
The Kidney Profile
The NKF Laboratory Engagement Plan recommended the development and industry-wide implementation of a "Kidney Profile," consisting of blood creatinine, eGFR, and uACR to improve screening and monitoring of patients at high risk for CKD (53). Results from the CAP 2020-Chemistry Survey showed that among US participants, only 15% offered the Kidney Profile (54). The Kidney Profile is aimed, in part, at increasing utilization of uACR, which is used in combination with eGFR for CKD diagnosis, classification, risk stratification, monitoring, and therapy. uACR is significantly underused (55-57). The reasons underlying underutilization are multifactorial, but include non-standardized reporting (54), and clinician uncertainty around test utility and interpretation (51, 58). The frequency of CKD monitoring with the Kidney Profile should be tailored to the underlying cause of CKD, the rate of change of eGFR or uACR, the presence of one or more clinical risk factors, changes to medication management, and intercurrent illness.
What equations were most commonly used for EGFR prior to the introduction of the CKD-EPI 2021 equations?
Key summary points:
- The most commonly used eGFR equations prior to 2021 were the 4v-MDRD and CKD-EPI 2009 equations, which both incorporate Black race coefficients.
- Race and ethnicity are imprecise, nebulously defined systems of classification as they pertain to genetic ancestry, physiological characteristics, and socioeconomic status, and therefore should not be used to classify individuals into distinct biological categories.
- The CKD-EPI 2021 refit equations were developed because a race-free, equitable approach to eGFR was desired and needed.
Creatinine has been used to assess GFR for over 100 years with the Cockcroft-Gault equation to estimate creatinine clearance becoming widely adopted in 1976 (59). The latter was considered a reasonable surrogate for evaluation of GFR and utilized to interpret pharmacologic data and establish medication dosing recommendations (60). In 1999, the 6-variable Modification of Diet in Renal Disease (6v-MDRD) study equation was published (61). This equation was developed from a predominantly White cohort of 983 men and 645 women enrolled in a clinical study to assess the potential effects of low protein diets on progression of CKD. The study evaluated 16 patient variables and subsequently derived equations to estimate GFR. The subsequent 4-variable MDRD equation, which also incorporates a Black race coefficient, has been widely validated. The studies used to generate the MDRD equations were not consistent in the way that race was assigned (61). The Kidney Disease Outcomes Quality Initiative (KDOQI) embraced the 4v-MDRD eGFR equation and recommended its use as a foundation for diagnosis and classification of CKD (44). Use of the Black race coefficient in these equations became widely accepted. Subsequently, automated reporting of eGFR was endorsed and adopted by clinical laboratories to help providers to interpret kidney function based on systemic creatinine concentration (21).
In 2009, the CKD Epidemiology Collaboration (CKD-EPI) derived an equation based on a pooled analysis of 10 studies. The equation was validated in 16 international cohort studies, which involved both mGFR and blood creatinine (62). These studies included individuals across a wide range of age, race, GFR, and creatinine concentrations. The resulting CKD-EPI 2009 equation exhibited improved performance, including greater accuracy and precision at higher GFRs as compared to the 4v-MDRD eGFR equation. However, similar to the 4v-MDRD equation, the derived CKD-EPI 2009 equation incorporated a Black race coefficient, albeit with a smaller modification coefficient (1.16 vs 1.21). The race coefficient gave a more accurate eGFR relative to mGFR in the cohorts used to develop and validate the CKD-EPI 2009 equation (62). As with the 4v-MDRD equation, the studies used to generate the equation were not consistent in the way that race was assigned. The 4v-MDRD remained the predominant equation used in the United States over the past decade (54).
Why is it problematic to include race as a demographic variable in medical algorithms, including estimated glomerular filtration rate (eGFR) equations?
Race, ethnicity, genetic ancestry, and consequently, genetic variants that influence disease and health outcomes, are inextricably linked; however, race and ethnicity are imperfect surrogates for genetic ancestry (63). Notably, African populations exhibit a significant degree of genetic diversity (64). This diversity combined with historic and ongoing admixture between persons of different ancestries within the United States has contributed to genetic divergence within racial groups (65, 66). No clinical gold standard exists to determine racial classifications (67). Race and ethnicity are self- or socially ascribed identities that are often inferred based on physical characteristics such as skin color (63). The definitions of race vary widely and have changed over time based on cultural and social contexts, geography, and geopolitical events (63, 67). While race and ethnicity may partially represent genetic ancestry, their use also highlights the effects of negative social determinants of health on racial and ethnic minority groups due to inequitable access to, and allocation of, health and social resources (4, 63). Racial and ethnic minorities in the United States are more likely to experience negative social determinants of health but are also socioeconomically diverse (68). The NKF-ASN Task Force acknowledged that the inclusion of race in the practice of medicine is challenging and problematic due to the complex and changing racial and ethnic makeup of persons (4).
The use of race and ethnicity in clinical algorithms and laboratory calculations may introduce disparities in healthcare, as race and ethnicity are social, rather than biological constructs (63, 64). Efforts over the last several years have intensified in recommending the removal of race and ethnicity from laboratory calculations and other medical algorithms, including eGFR equations, due to concerns that their inclusion appears to endorse a biological basis for race (69). There are racial and ethnic disparities in both kidney health and healthcare that are influenced by social, environmental, and biological factors (4). Black Americans have a higher prevalence of kidney failure and are less likely to receive patient-centric kidney failure replacement therapies, including home dialysis, and kidney transplantation, as compared to non-Hispanic White Americans (14). In the development of the 4v-MDRD and CKD-EPI 2009 equations, coefficients were included in calculating eGFR in Black patients to account for higher serum creatinine concentrations observed in Black patients relative to their mGFRs and to improve accuracy (40, 61, 62). These equations produce a higher eGFR value in Black individuals compared to non-Black individuals (61, 62). This practice has the potential to introduce systematic differences in care between races (4, 5). For example, studies have shown that use of the Black race coefficient could result in delayed achievement of a clinical threshold for kidney transplant referral and eligibility in Black patients, compared to removal of the Black race coefficient (70, 71).
While studies have reported that the proportion of African ancestry found in an individual positively correlates with serum creatinine concentration, a similar association between African ancestry and mGFR has not been demonstrated (72, 73). Although equations utilizing a Black race coefficient were rapidly adopted in the United States, multiple studies conducted in Black populations outside the United States demonstrated limited evidence for the appropriate use of these coefficients in eGFR equations. A recent systematic review utilized an evidenced-based approach to examine the utility of Black race coefficients in eGFR equations in African and Brazilian populations (74). Across 10 studies representing 1749 participants that directly compared mGFR to the 4v-MDRD or CKD-EPI 2009 eGFR equations, exclusion of the Black race coefficient led to improved agreement with mGFR in Black persons (74). Furthermore, in studies conducted in the United States and the United Kingdom, inclusion of Black race coefficients in estimating equations led to eGFR results that were discordant with markers of KD-related metabolic dysfunction (e.g., secondary hyperparathyroidism), and overestimation of eGFR relative to mGFR in prospective kidney donors (74).
In summary, race and ethnicity are imprecise, nebulously defined systems of classification as they pertain to genetic ancestry, physiological characteristics, and socioeconomic status (8). As such, use of race or ethnicity in medical algorithms such as estimating GFR is fraught with difficulty and may lead to unintentional bias that affects clinical care.
What are the new equations and how were they derived?
Key summary points:
- The CKD-EPI 2021 equations are listed in Table 2, and were derived in a diverse cohort of participants with respect to age, sex, BMI, and GFR, in which race was mostly self-reported.
- The CKD-EPI 2021 eGFRcr equation performs similarly to the CKD-EPI 2009 equation with respect to the percentage of measured GFR values within ±30% of the corresponding eGFR value (P30) and CKD classification. The P30 of the CKD-EPI 2021 eGFRcr equation is 87% in Black individuals and 86% in non-Black individuals. The P30 of the CKD-EPI 2009 eGFRcr equation is 85% in Black individuals and 89% in non-Black individuals.
- The CKD-EPI 2021 eGFRcr equation underestimates GFR in Black individuals by 3.6 mL/min/ 1.73 m2 and overestimates GFR in non-Black individuals by 3.9 mL/min/1.73 m2.
- The CKD-EPI 2021 eGFRcr-cys equation underestimates GFR in Black individuals by 0.1 mL/min/ 1.73 m2 and overestimates GFR in non-Black individuals by 2.9 mL/min/1.73 m2.
- The uncertainty in eGFR values is large and similar between CKD-EPI 2009 eGFRcr and CKD-EPI 2021 eGFRcr equations with root mean square errors (rmse) of 0.192 and 0.201, respectively.
- The uncertainty in eGFR values is less for 2021 eGFRcr-cys(rmse 0.177) compared to the 2021 eGFRcr equation.
The CKD-EPI 2021 equations are listed in Table 2. The equations were derived using the same data pools used in the original derivation of CKD-EPI 2009 eGFRcr equation. The CKD-EPI 2009 development data set consisted of 10 studies with a total of 8254 participants, and the CKD-EPI 2012 development data set (eGFRcys and eGFRcr₋cys) consisted of 13 studies with a total of 5352 participants (39). For both CKD-EPI 2021 equations, the regression function that was used for the 2009 and 2012 equations was used to fit new models that excluded race as an explanatory variable. The equations were validated in a pooled analysis of 12 studies comprising 4050 participants with and without CKD, who self-reported as Black or non-Black in most studies. Approximately 13.6% of the US population is Black (75). Black participants accounted for 31.5% of the 2009 development data set, 39.7% of the 2012 development data set, and 14.3% of the 2021 validation data set (39).
The CKD-EPI 2021 eGFRcr equation performed similarly to the CKD-EPI 2009 equation with respect to the percentage of measured GFR values within ±30% of the corresponding eGFR value (P30) and assignment of GFR classifications. The rmse is commonly used to evaluate how far predictions (e.g., eGFRs) fall from measured true values (e.g., mGFRs). The uncertainty in eGFR values was large and similar between CKD-EPI 2009 eGFRcr and CKD-EPI 2021 eGFRcr equations with rmse of 0.192 and 0.201, respectively. Whereas the CKD-EPI 2009 equation overestimated GFR in Black participants by 3.7 mL/min/1.73 m2, the CKD-EPI 2021 eGFRcr equation underestimated GFR by 3.6 mL/min/1.73 m2. The magnitude of bias in non-Black participants increased to an overestimate of 3.9 mL/min/1.73 m2 with the CKD-EPI 2021 eGFRcr equation, compared to 0.5 mL/min/ 1.73 m2 with the CKD-EPI 2009 equation. The CKD-EPI 2021 eGFRcr₋cys equation performed similarly to the CKD-EPI 2012 eGFRcr₋cys equation with respect to P30 and assignment of GFR classification with an underestimate of 0.1 mL/min/1.73 m2 in Black participants relative to the overestimate of 2.5 mL/min/1.73 m2 observed with the CKD-EPI 2012 eGFRcr₋cys equation. In non-Black participants, overestimates of 0.6 and 2.9 mL/min/1.73 m2 were observed using the CKD-EPI 2012 eGFRcr₋cys and CKD-EPI 2021 eGFRcr₋cys equations, respectively. The uncertainty in eGFR values is less for 2021 eGFRcr₋cys (rmse 0.177) compared to the 2021 eGFRcr equation (rmse 0.201) (39).
The NKF-ASN Task Force recommended immediate implementation of the CKD-EPI 2021 eGFRcr equation (5). The CKD-EPI 2021 eGFRcr equation was developed in a diverse cohort with a similar percentage of Black individuals as the US population. The equation exhibits performance characteristics that are acceptable for clinical use, does not disproportionately affect any one group of individuals, and achieves the goal of eliminating the use of race in estimating GFR. Immediate implementation of the CKD-EPI 2021 eGFRcr equation is feasible as creatinine is measured in most clinical laboratories and the structure of the 2009 and 2021 equations are identical, although some of the numeric variables/coefficients differ. Given the improved performance achieved through use of both cystatin C and creatinine, the Task Force also recommended increased use of the CKD-EPI 2021 eGFRcr₋cys in cases where eGFRcr may not provide accurate estimates.
How can the clinical Laboratorian contribute toward closing racial/ethnic disparities in CKD?
Key summary points:
- Early detection and awareness of KD in clinically and socioeconomically high-risk populations is critical to achieving equitable kidney care.
- Laboratorians can contribute toward closing racial/ethnic disparities in CKD through:
- Standardization of CKD biomarker testing and eGFR reporting,
- Optimization of CKD biomarker test utilization and interpretation,
- Avoiding the use of Jaffe reactions and instead using enzymatic measurement of creatinine assays, and
- Providing cystatin C testing with a short turnaround time.
More than 90% of individuals with CKD are unaware of their disease and almost half are in advanced stages when they receive a definitive diagnosis (76). Advocacy efforts have focused on earlier detection, practitioner recognition, and patient awareness of KD, as these provide opportunities for clinical and lifestyle interventions that can slow CKD progression, but remain a significant challenge (58). Racial and ethnic disparities in KDalso include late referral for nephrology care, specifically in younger Medicaid beneficiaries of low socioeconomic status, which highlights the importance of screening in the primary care setting (9, 58). The achievement of equity in kidney care will require key stakeholder collaboration to increase early detection and awareness of KD in clinically and socioeconomically high-risk (high SDI) populations (77).
As diagnosis and classification of CKD are based on laboratory testing, laboratorians are well-poised to participate in efforts to improve CKD recognition through standardization of CKD biomarker testing and reporting (8, 44), optimization of CKD biomarker test utilization and interpretation, and their application to population health initiatives (77, 78). These efforts must be executed in collaboration with interdisciplinary clinicians across the kidney care continuum, align with nationally recommended CKD quality objectives and metrics (79-84), and be outcome driven (79).
Guidelines and recommendations for standardization of testing and reporting for creatinine, cystatin C, uACR, and eGFR are listed in Table 4 (8, 23, 44). Efforts to improve utilization of kidney screening tests should focus on increasing targeted screening of high-risk populations, particularly in primary care settings, at least annually using eGFR and uACR combined or within the Kidney Profile (8, 44, 77, 85). Clinical laboratories can improve test interpretation for both eGFR and uACR by linking guideline-defined GFR and albuminuria CKD categories with test results. Alternatively, these can be listed directly on the report. A hyperlink can be provided to the categories for systems incapable of complex result comments. The Kidney Profile (eGFR and uACR) should be offered as a separate, distinct test from a Renal Function Panel (blood albumin, urea nitrogen, sodium, calcium, carbon dioxide, chloride, creatinine, glucose, phosphorus, potassium), which is an American Medical Association-recognized test panel that is better suited for monitoring patients with established CKD (51). Near patient testing and direct-to-consumer/direct access testing may offer advantages to traditional approaches in some instances to reach high-risk groups (86-88).
Clinical laboratory leaders can significantly contribute to decreasing the racial and ethnic disparities in CKD by developing multidisciplinary kidney quality improvement initiatives that include characterizing the populations served and unserved, identifying testing strategies that align with expert guidelines, and including appropriate test menus and clinical decision support tools within their healthcare systems. Laboratory personnel can also advocate (e.g., at the local, state, national, and professional levels, and medical and clinical pathology societies) for care for uninsured patients, since lack of insurance is an independent risk factor for early death and ESKD in patients with CKD (89). Several healthcare systems have implemented kidney quality improvement initiatives and reported positive screening and patient outcomes that include increased uACR testing, improved CKD recognition, increased nephrology referrals and reduced hospitalizations (90-93). For example, one system implemented a "creatinine safety program" to increase follow-up evaluation of all single abnormal creatinine results recorded in the electronic health record (EHR), since diagnosis of CKD requires establishing chronicity (93). The EHR was used to identify patients with abnormal creatinine results that did not have repeat creatinine evaluation within 90 days, and these patients were then contacted to coordinate repeat testing. This initiative led to 3668 CKD diagnoses and 336 nephrology consults within 6 months (93). Laboratories can also leverage EHR and laboratory information system (LIS) data to measure the impact of KD interventions, e.g., implementing race-neutral eGFR equations, on patient kidney health and outcomes. Health record-based CKD registries that identify patients with CKD based on laboratory data to target interventions have improved clinical outcomes (94,95).
While expert panels currently recommend against screening in the general population in favor of targeted testing for CKD among high-risk populations (44,85), laboratory data collected during routine care, urgent care, or emergency department visits can provide early, clinically actionable insight as seen in the "creatinine safety program" example (93). Creatinine is measured in basic and comprehensive metabolic panels, and eGFR is reported in 92% of clinical laboratories (54). This provides a rich source of data for identifying people at risk of CKD. Patient results can:
- be flagged and/or annotated using LIS and/or middleware rules;
- trigger clinical decision support tools if the results meet guideline-defined criteria for CKD diagnosis (eGFR < 60 mL/min/1.73 m2) for 3 or more months
- trigger clinical decision support tools if the results meet guideline-defined criteria for referral to nephrology including:
- GFR< 30 mL/min/1.73 m2,
- a decline in GFR category accompanied by a ≥ 25% drop in eGFR from baseline,
- a decline in eGFR of >5 mL/min/1.73 m2/ year,
- uACR > 300 mg/g (consider referral if unexplained), and
- uACR > 2000 mg/g(nephrotic range albuminuria).
How should CKD-EPI 2021 equations be deployed by clinical laboratories?
Key summary points:
- Calculations from programmed and preprogrammed CKD-EPI 2021 equations must be extensively verified for mathematical accuracy compared to reference programming across different creatinine concentrations, ages, and sexes.
- eGFR can be reported as a numeric value >60 mL/min/1.73 m2 when calculated using the CKD-EPI 2021 equations.
- eGFR results should include a comment or should be named to indicate which equation was used, for example “eGFR was calculated from creatinine using the 2021 CKD-EPI equation.”
- CKD-EPI 2021 eGFRcr and eGFRcr-cys should not trend with results from older equations.
The NKF Laboratory Engagement Working Group and CKD-EPI provide comprehensive guides for implementation of the CKD-EPI 2021 equations (23, 96). Reporting recommendations are detailed in Table 2.
General programming instructions for the equations are included in Supplemental Table 1. Of note, several LISs provide the CKD-EPI 2021 eGFRcr equation in their foundational programming, making it more feasible for laboratories to transition to the new equation. All LIS vendors should offer updated equations as ready-to-use, thereby alleviating laboratories of the need to con- duct site-specific programming and further aiding in standardization of result reporting. However, even with the availability of pre-programmed equations in the LIS or middleware solutions, laboratories should carefully verify the accuracy of values calculated by these equations. This may be achieved by calculating eGFR using the CKD-EPI equations in patients with different creatinine and cystatin C concentrations, sexes, and ages, and com- paring the results with those derived from calculators provided by the NKF. Online calculators and mobile applications created or endorsed by the NKF may be used during equation performance verification (97). It is also recommended that laboratories test the correct flagging of abnormal results and correct triggering of testing algorithms (e.g., reflex testing), as appropriate. Of note, the NKF has created a table with different conditions for testing CKD-EPI 2021 eGFRcr and CKD-EPI 2021 eGFRcr-cys equations (23). KDIGO recommends that eGFR values <60 min/ mL/1.73 m2 should be reported as decreased, how- ever, diagnosis of CKD requires establishing chronicity of decreased GFR or markers of kidney damage, such as albuminuria, to distinguish chronic from acute KD (8, 44). Therefore, clinical context and previous eGFR values must be considered to guide appropriate follow-up. Further, the values that should be flagged as abnormal may vary depending on the patient population being served, (e.g., in- patient vs outpatient, since unstable kidney function in inpatients may be difficult to assess). Most importantly, primary care providers and nephrologist must be familiar with institution-, department-, or site- specific flagging rules.
Laboratories should carefully design the reporting of the results derived from the revised race-agnostic eGFR equations to facilitate the correct interpretation of results by healthcare providers and patients. Re-baselining (aka parallel testing) across the new and old equations is not necessary. The concentration of creatinine can be in- formative in detecting changes over time (98). Reporting of eGFR should be standardized, and it is recommended that eGFR is reported as a whole number in units of mL/min/1.73 m2. The historic upper limit of eGFR reporting was 60 mL/min/
1.73 m2 due to poor performance of the 6v- and 4v-MDRD equations at higher GFRs. With improved performance of CKD-EPI equations, including the CKD-EPI 2021 refit equations, it is recommended that eGFR values >60 mL/min/1.73 m2 be reported as a numeric value to support early detection of declining kidney function (23). For example, a sustained decline in eGFR of >5 mL/min/1.73 m2/year war- rants investigation (8, 99). Furthermore, there are patient populations in which hyperfiltration may be observed e.g., critically ill patients, or diabetic patients, where an abnormally high eGFR may prompt uACR measurement (100). eGFR values corresponding to the lower limit of creatinine reporting can be reported, but the limited accuracy of these estimates relative to mGFR must be considered. A recent cross-sectional study quantified the magnitude and consequences of individual-level differences between mGFR and eGFR, using data from 4 community-based prospective cohort studies representing a total of 3,223 participants (101). While population level differences between mGFR and CKD-EPI 2021 eGFRcr (mGFR-eGFRcr) were relatively small at −0.6 mL/min/1.73 m2, individual-level differences between mGFR and eGFRcr were relatively larger and increased with increasing eGFR (101). The range of distributions of mGFR at each eGFR value examined was narrower for both the CKD-EPI 2021 eGFRcr-cys and the CKD-EPI 2012 eGFRcys equations compared to the CKD-EPI 2021 eGFRcr equation (101). Clinical laboratories can append reports with comments reminding providers of the limited accuracy of eGFR for individual patients (102).
Although rebaseline testing is not necessary, when implementing the CKD-EPI 2021 eGFRcr and/or CKD-EPI 2021 eGFRcr-cys equations, results should not be trended with results from different and older equations. This may be accomplished by building re- fit equations as new tests or test components and displaying the results in unique new rows within the electronic medical record. The CKD-EPI 2021 equations have distinct LOINC codes and should be resulted in distinct result fields to allow for the correct LOINC code to be applied overtime (103). When applicable, healthcare systems should work to reduce complexities associated with receiving eGFR results from outside laboratories. Equation-specific resulting names or interpretive comments should be utilized to notify providers of the equations used to estimate GFR. Sample report comments are avail- able on the NKF website and can be modified to meet the needs of the laboratory, health care professionals, and patients (96).
Laboratories should also consider creatinine measurements from point-of-care (POC) testing devices, as not all POC devices have the capability to report eGFR using the 2021 refit equations. POC devices used to measure creatinine should use measurement procedures with calibration traceable to isotope dilution mass spectrometry, and have the capability to report eGFR using equations recommended by professional societies. If a POC device does not have the ability to align with central laboratory testing, whether in terms of absolute creatinine concentration, creatinine reporting units, or eGFR equations used, results should not be trended in the medical record with central laboratory results. POC devices typically have poorer precision, so the uncertainty of creatinine and eGFRcr will be larger, and may influence a decision to trend POC results with those from central laboratory.
What changes can be expected in patient management, drug dosing, and transplant eligibility by implementing the CKD-EPI 2021 EGFRcr equation?
Key summary points:
- Implementation of the CKD-EPI 2021 eGFRcr equation will lead to a lower eGFR in Black individuals and higher eGFR in non-Black individuals compared to the CKD-EPI 2009 equation that included race.
- When the eGFR flanks a clinical decision point, confirmatory assessment can be performed using direct measurement of glomerular filtration, measurement of creatinine clearance, serial creatinine-based measurements, or estimation of GFR including cystatin C.
Removal of the Black race coefficient and transition to the new CKD-EPI 2021 eGFRcr equation will predictably lead to a lower eGFR in individuals in whom the Black race coefficient was previously applied, and an increased eGFR in those for whom it was not. Combined, changes to the calculation for eGFR will alter CKD classification in patients where eGFR was close to clinical decision thresholds (104, 105).
Across the spectrum of eGFR values, transition to the new equations yields a range of considerations. In individuals with an eGFR close to normal, a shift to the race-neutral equation only impacts potential kidney donor candidates whose eGFR crosses the threshold used at their transplant center. For these individuals, the shift to the new equation may prevent harm to a potential donor since the CKD-EPI 2009 equation (inclusive of the Black race coefficient) may have overestimated GFR in potential Black donors (104, 106). Further, use of a CKD-EPI 2021 equation may instead prompt appropriate evaluation for KD, such as screening for albuminuria. However, when eGFR is near (above or below) the threshold used to permit donation at a transplant center, shift to the new equation could lead to harm by preventing kidney donation in a potentially eligible donor be- cause of underestimation of GFR. This can be mitigated by comparing the eGFRcr to the eGFR calculated with the CKD-EPI 2021 eGFRcr-cys equation along with an assessment for albuminuria to ensure the safety of kidney donation. The eGFR is also used to identify patients that are eligible to list for deceased donor preemptive kidney transplant. Although most preemptive transplants come from living donors, potential recipients are typically not referred to a transplant center until they have an eGFR of ≤20 mL/min/1.73 m2. The CKD-EPI 2009 equation has the potential to delay evaluation (70). Based on these concerns, the Federal Organ Procurement Transplantation Network (OPTN) endorses a race-neutral assessment of GFR (107).
Many medications and metabolites are excreted by the kidney and a change in eGFR may prompt concerns regarding drug dosing. Since eGFR is embedded in current clinical practice, the US FDA recommends use of eGFR with any “contemporary, widely accepted and clinically applicable estimating equation for the population studied” (108). Dosing parameters are of particular concern with traditional chemotherapeutic agents, antibiotics, contrast agents for radiology, and medications used to treat diabetes mellitus. Using eGFR to delineate who is eligible for a particular drug and define the appropriate dose has the potential for “underdosing,” (i.e., inappropriate cessation of a medication or inappropriate agent exclusion if the eGFR is an underestimate of the true GFR) and “overdosing” (i.e., toxicity if the eGFR is an overestimate of kidney GFR). This is particularly salient for eGFRs at the decision points of 60 and 30 mL/min/1.73 m2, which define stages G3a and G4 of CKD, respectively. When the eGFR flanks a clinical decision point, providers may consult with a nephrologist or pharmacist for support in dosing considerations. In addition, confirmatory assessment can be performed using estimation of GFR including cystatin C, direct measurement of glomerular filtration rate, measurement of creatinine clearance, or serial creatinine-based measurements. eGFR can be de-indexed by multiplying the indexed eGFR in mL/min/m2 by the patient’s BSA in m2 and then dividing by 1.73. Notably, when using eGFR for medication dosing, the eGFR value should be de-indexed (converted to actual mL/min) from BSA. This is particularly important in individuals at extremes of weight, as drug clearance is related to total eGFR not indexed eGFR.
How should changes to EGFR reporting be communicated?
Key summary points:
- All clinical care providers should be informed of the implementation of the CKD-EPI 2021 equations.
- Communications should emphasize what changes should be expected and encourage providers to interpret eGFR based on clinical context, given the limitations of eGFR as an estimate of GFR
Implementation requires communication with all stakeholders who care for adults. Collaboration between clinical laboratories, nephrologists, and other subject-matter experts can achieve broad coverage and dissemination of information. Although pharmacists and those practicing internal medicine may be the most affected, those practicing radiology, those who or- der contrast-based imaging, transplant surgeons, and providers who prescribe medications that are cleared by the kidney, such as antibiotics, lithium, and antiepileptic agents, also need to be aware of the change. Institutional communication should include provider-wide and redundant approaches to maximize the likelihood of information reaching all caregivers. Communications should be explicit and provide an educational basis, outlining the new equation and how results will be affected. Educational material should high- light that the eGFR is only an estimate rather than a measured value. The 2021 CKD-EPIcr P30 is roughly 86%, meaning that 14% of eGFR values were >±30% of the measured GFR in the study cohort (39). Indeed, eGFR values perform well at a population level but for an individual, the inaccuracy of the estimate needs to be considered (101). Last, the educational content should reinforce that the eGFR is designed to estimate kidney function when patients are medically stable and cannot be used when the kidney function is changing, such as with AKI (23).
When should eGFR equations including cystatin C be used?
Key summary points:
- eGFR calculated using the CKD-EPI 2021 eGFRcr-cys equation is generally more accurate compared to eGFR calculated with the CKD-EPI 2021 eGFRcr equation, and should be used when eGFR is close to a clinical decision point where higher accuracy is required.
- In cases where creatinine is confounded by non-GFR determinants (Table 3), an estimate calculated using the CKD-EPI 2012 eGFRcys equation is preferred.
- Cystatin C has non-GFR determinants (Table 3), which may impact the accuracy of eGFR equations that incorporate cystatin C.
Cystatin C testing may be complementary in individuals with low creatinine production, where creatinine-based eGFR overestimates true GFR, such as individuals with sarcopenia, amputees, as well as those who are frail and elderly (36, 109). Cystatin C testing is also recommended in individuals where creatinine production is in- creased and serum creatinine-based eGFR underestimates true GFR, such as body builders and other individuals who exercise vigorously and have increased muscle mass, individuals with high exogenous creatine ingestion, and anabolic steroid users (36, 109). Use of the CKD-EPI 2021 eGFRcr-cys equation may offer more accurate estimates near eGFR clinical decision points (23, 39); however, cystatin C has non-GFR determinants (Table 2), which must be considered when choosing which eGFR equation may provide the best estimate of GFR (25, 36). eGFRcys may be more accurate than eGFRcr-cys in patients with large effects of non-GFR determinants of creatinine.
Increased adiposity is associated with increased levels of circulating cystatin C, and one study found that equations that incorporated both cystatin C and creatinine (CKD-EPI 2012 eGFRcr-cys) showed reduced bias relative to mGFR compared to cystatin C (CKD-EPI 2012 eGFRcys) or creatinine-only (CKD-EPI 2009 eGFRcr) equations in a cohort of 166 obese CKD patients (25). In a small cohort (n = 66) of patients with chronic heart failure, eGFR calculated with cystatin C (CKD-EPI 2012 eGFRcys) exhibited a bias of −4.1 mL/min/1.73 m2 relative to mGFR (26). eGFR calculated with creatinine (CKD-EPI 2009 eGFRcr) or creatinine and cystatin C (CKD-EPI 2012 eGFRcr-cys) exhibited biases of −15.2 mL/min/1.73 m2 and −7.8 mL/min/1.73 m2 relative to mGFR, respectively (26). Further, the P30 for eGFRcys in the cohort of patients with chronic heart failure was 65% com- pared to that of eGFRcr, which was 33%, and eGFRcys agreed more closely with mGFR in classifying patients in to CKD Stages 3, 4, and 5 compared to eGFRcr and eGFRcr-cys (26). eGFRcys (CKD-EPI 2012 eGFRcys) and eGFRcr-cys (CKD-EPI 2012 eGFRcr-cys) have been found to be more accurate compared to eGFRcr (CKD-EPI 2009 eGFRcr) in patients with liver cirrhosis, but both equations were less accurate at lower GFRs (27, 28).
KDIGO 2012 guidelines recommend cystatin C testing for dosing medications with narrow therapeutic indices, such as vancomycin, aminoglycosides, and chemotherapeutic agents (8, 110). A systematic review examined the use of eGFR equations that incorporate cystatin C for drug dosing across 34 studies with a total of 3455 participants and 16 different medications (111). In most studies, eGFRcys was a better predictor of drug levels and clearance compared to eGFRcr (111). eGFRcr-cys was only assessed in 5 studies and showed superior performance to equations incorporating either biomarker alone (111).
In patients where both creatinine and cystatin C may be influenced by non-GFR determinants, mGFR or creatinine clearance should be used at clinical decision points and for dosing of nephrotoxic medication and medications with a narrow therapeutic index (34). Large differences between eGFRcys and eGFRcr (eGFRdiffcys-cr = eGFRcys-eGFRcr) indicate that non-GFR determinants are causing a substantial change in one of the biomarkers (112). Approximately 33% of participants in the Chronic Renal Insufficiency Cohort Study, a multicenter observational cohort study of 5499 adults from 7 clinical centers across the United States, had eGFRdiffcys-cr≥15 mL/min/m2 (112). In this setting, eGFRcr-cys was generally more accurate than either eGFRcr or eGFRcys, but reporting eGFRcr-cys without reporting eGFRcr or eGFRcys separately could potentially obscure the influence on non-GFR determinants (113). Importantly, eGFRdiffcys-cr, is also prognostic of ESKD, mortality, hospitalization, and cardiovascular disease (34). Clinical judgment based on patient- specific factors should be exercised in patients with discrepant eGFRcr, eGFRcr-cys, and eGFRcys results who may benefit from a more global assessment of kidney function.
What challenges are associated with implementing cystatin C testing?
Key summary points:
- Cystatin C testing should be offered by clinical laboratories to facilitate calculation of eGFR using both creatinine and cystatin C.
- Implementation of cystatin C testing should be accompanied by institutional practice guidelines or educational initiatives, annotation of results with interpretive and educational comments, and clinical decision support or reflex testing to aid provider utilization and interpretation.
There are barriers to the widespread implementation of cystatin C testing in clinical laboratories (5). In the 2019 CAP survey of 3900 US respondents, only 2% reported offering cystatin C in- house, compared to 90% that sent specimens to reference laboratories for testing and 8% not answering the question (23). Reference laboratory cystatin C testing is a viable option to facilitate testing demands for CKD diagnosis and management as long as they report the results with the appropriately calculated eGFR. Reference laboratory cystatin C testing may present a challenge for use in AKI and emergent settings where a shorter turnaround time is required (41, 42, 110). Cystatin C results from a referral laboratory should be reported with an accompanying eGFR incorporating the biomarker. Cystatin C testing can be performed on most high-throughput automated chemistry analyzers and assay harmonization has considerably improved. In the CYS-B 2022 survey, which was the most recent cystatin C CAP survey at the time of this report, method-specific means ranged from −9.5% to 14% around the all-method mean, compared to 2014 when they ranged from −12% to 29% (70, 71). Several scalability challenges to cystatin C test implementation exist. First, measurement of cystatin C relies predominantly on immunoturbidimetric approaches, in contrast with creatinine, which is measured using enzymatic or colorimetric assays that are more rapid and cost-efficient. Incorporation of cystatin C into basic and comprehensive metabolic panels to enable routine calculation of eGFRcr-cys and eGFRcys may be impractical, due to the significantly increased volume of cystatin C reagent that would be required, as vendors work to sustainably increase production. Another scalability challenge centers around a lack of clinical decision support. Currently, decision support on when to perform cystatin C testing for clinical workflows is not standardized.
The increased cost of cystatin C compared to creatinine is often cited as barrier to widespread implementation (109, 114, 115). However, cost may decrease with more widespread implementation and increased test volumes (109). The differential cost is also reflected in the higher CMS (Centers for Medicare and Medicaid Services) 2022 reimbursement rate for cystatin C ($18.52) vs creatinine ($5.12) (116). Comparative reimbursements for the basic and comprehensive metabolic panels that are used more frequently than creatinine ordering alone are $8.46 and $10.56, respectively. The NKF-ASN Task Force highlighted the need for changes in Current Procedure Terminology (CPT) coding to encourage use of cystatin C (5). Currently, incorporation into basic and comprehensive metabolic panels would also be a cost burden on the patient without pay or support. Data on hospital/system-wide cost-savings, if any, that may be realized with more accurate cystatin C-based eGFR estimates are lacking. As healthcare transitions from fee-for-service to value-based care, use of cystatin C-based eGFR estimates may become more widespread in spite of cost, if use of cystatin C can improve patient outcomes through better risk stratification and interventions. Lack of provider familiarity with cystatin C result interpretation, lack of knowledge of non-GFR determinants of cystatin C and absence of clinical practice guidelines represent additional barriers to widespread utilization (110). Interdisciplinary collaboration between nephrology and the clinical laboratory may help to overcome these challenges through development of institutional practice guidelines or educational initiatives, annotation of results with interpretive and educational comments, and clinical decision support or reflex testing for patient populations in which cystatin C-based eGFR calculations are more appropriate as described before.
How do sex and gender influence eGFR equations?
Key summary points:
- In transgender, nonbinary, or intersex people, eGFR should be evaluated using both the male and female constants with CKD-EPI 2021 equations. Considering both values is particularly relevant at the onset of CKD and/or when approaching important thresholds.
- When eGFRcr calculated with either sex constant crosses a clinical threshold, a holistic approach should be taken to determine appropriate management anchored to the muscle mass of the individual, and based on their sex hormone configuration and gender identity.
- More data is needed on the impact of gender- affirming hormones on cystatin C, and the use of cystatin C-based eGFR estimates in gender-diverse populations.
Equations to estimate GFR include binary dependent variables that classify individuals as male/female or as a man/woman (39). These variables were included to account for the apparent differences in muscle mass between females and males and were supported by the observed biases between mGFR and eGFR. The difference between male and female variables is larger for eGFRcr than for eGFRcys, and intermediate for eGFRcr-cys. Gender and differences in sexual development (intersex), however, were not directly included in the development or validation of eGFR equations and may influence muscle mass through diet and behavior, or variance in sex hormone administration or expression.
Increasing societal and cultural recognition of gender variance complicates the use of eGFR equations and our ability to classify humans based on perceived sex. In contrast to sex, which is biologically defined based on the visual appearance of external genitalia at birth, and/or in ambiguous cases, the presence or absence of a Y chromosome, gender identity encompasses the psychosocial characteristics that define an individual’s identity or expression as masculine, feminine, or nonbinary (117). Cisgender people have a gender identity that aligns with their sex assigned at birth; transgender or gender-diverse people have a gender identity that is incongruent with their sex assigned at birth. A transgender man was assigned female sex at birth and identifies as a man; a transgender woman was assigned male sex at birth and identifies as a woman; a nonbinary person was assigned male or female sex at birth and may identify as both a man or a woman or as neither. Intersex individuals have an array of underlying mechanisms for their phenotypic differences that are either developmental and/or genetic mutations due to in utero exposure of sex hormones, metabolites in sex hormone synthesis, androgen insensitivity, or to other unusual transcription factors or receptors. Transgender people may be intersex, but people who are intersex are not necessarily transgender. Any of these gender-diverse individuals may present as androgynous, masculine, feminine, or fluctuate across the spectrum. Medical care for transgender and nonbinary people may include gender-affirming hormones - testosterone and estradiol (with or without androgen blockade or progesterone), which are prescribed to promote development of masculinizing and feminizing secondary sex characteristics, respectively. The introduction of gender-affirming hormones will promote physiological changes that align with gender identity, including redistribution of fat and changes in muscle mass, and hence complicate the use of sex-specific constants in eGFR equations. Additionally, some transgender and nonbinary people will undergo gender-affirming gonadectomies, which may further mediate sex hormone concentrations and the downstream tissues they influence. Not all transgender people seek medical intervention and may appear as their affirmed gender even without hormones. Visual identification is an inappropriate mechanism for identifying gender-diverse people. Primarily, this is stigmatizing and “others” people, but also practically, pathologies such as polycystic ovarian syndrome and congenital adrenal hyperplasia may impose gender-diverse characteristics without accompanying a transgender identity.
A recent systematic review and meta-analysis of all studies related to eGFR in transgender people confirmed that serum creatinine concentration variably changes as a person transitions to their affirmed gender identity when using gender-affirming therapies (118). Specifically, after roughly 12 months on testosterone hormone therapy, creatinine concentrations increased by roughly 0.15 mg/dL (95%CI 0.00–0.29 mg/dL) in transgender men. In contrast, after a similar time frame, transgender women on estrogen hormone therapy do not show a statistically significant increase or decrease in creatinine concentration (average change from baseline −0.05 mg/dL; 95% CI −0.16–0.05 mg/dL). Importantly, the wide confidence intervals observed across the genders indicate that the average change in creatinine is not necessarily representative of the change at the individual level. The mechanism underlying the change (or lack thereof) in creatinine concentration is not defined, although it is hypothesized to result from changes in muscle mass and not GFR or tubular secretion. GFR was not measured in these studies, so this remains speculative. The authors did not find any literature whereby mGFR and eGFR were evaluated in transgender people, making it difficult to distinguish which sex- variable or alternate variable, if any, would allow for a more accurate estimation of GFR calculated by the currently available equations.
Until additional data are available, regardless of hormone therapy or other intervention use, we recommend evaluating eGFR using both the male and female constants with the CKD-EPI 2021 equations in transgender, nonbinary, or intersex people. If either of these results crosses a clinical threshold a holistic approach should be taken to determine appropriate management anchored to the muscle mass of the individual based on their sex hormone configuration and gender identity. uACR and 24-hour urine creatinine clearance measurements may provide more information on kidney function in gender-diverse individuals. Mathematically, the higher the eGFR, the larger the difference between eGFR (male) and eGFR (female) (119); however, considering both values is still relevant at the onset of CKD and/or when approaching important thresholds such as for transplant referral, dialysis initiation, or dosing of medications with narrow therapeutic indices. Until interfacing between the EHR and the laboratory information systems improve, there are limited automated informatics solutions to identify gender-diverse people and report both eGFR values (120). Data illustrating the impact of gender- affirming therapy on cystatin C are lacking (118), however, since cystatin C is less influenced by muscle mass (37), cystatin C-based GFR estimates could, in theory, improve screening for CKD or monitoring for CKD progression. Assessment of gender in the context of eGFR is an area for shared decision-making and an evolving area for investigation.
Additional considerations and outstanding gaps
Guidance on the Use of Cystatin C
Expert practice guidelines on cystatin C are needed to facilitate its increased use in clinical practice. The use of cystatin C in conjunction with creatinine can improve GFR estimates, however, in contrast to creatinine, providers are less familiar with indications for cystatin C-based eGFR calculations and result interpretation. Additionally, the non-GFR determinants of cystatin C are relatively less studied (37, 121). Calculation of eGFR using CKD-EPI equations based on different biomarkers (creatinine only, cystatin C only, or creatinine and cystatin C) may yield different, and at times contradictory, results in certain patient populations (e.g., the elderly) or when non-GFR determinants are important factors (121). Improved understanding of non-GFR determinants of cystatin C can be used to develop algorithms to support decision-making when there is discordance between estimates that incorporate cystatin C vs those based on creatinine alone.
Consensus Screening Guidelines
United States Preventative Services Task Force (USPSTF), the government agency responsible for outlining evidence-based guidelines for preventative medical services, has not issued recommendations for CKD screening (122). This is despite the high prevalence of CKD, low rates of detection, and current evidence supporting the need for screening of high-risk individuals. While the NKF KDOQI, KDIGO, and the American Diabetes Association recommend CKD screening using eGFR and uACR in high-risk individuals, the development of USPSTF CKD screening guidelines would streamline CKD testing strategies nationally, and will be critical in achieving health equity in KD. The development of consensus critical action and delta values for eGFR and uACR represent additional opportunities for improvement of CKD detection.
Novel Kidney Disease Biomarker Discovery
Notwithstanding the clinical utility of eGFR, it must be emphasized that eGFR is an estimate with multiple contributory sources of uncertainty, including uncertainty in mGFR, analytical uncertainty associated with measurement of creatinine and cystatin C, and biological variation (123). Research into novel endogenous filtration markers and KD biomarkers is needed to improve KD diagnosis, management, and treatment (5). Initiatives such as the Kidney Precision Medicine Project (KPMP) (33) seek to better define the molecular underpinnings of both CKD and AKI, with the goal of KD biomarker discovery, and the development of novel therapeutics with companion diagnostics. The findings of the KPMP and similar initiatives have the potential to enable precision medicine for KD. Integration of disparate data sources such as clinical imaging, cellular data, proteomic data, and genomic data, through EHR systems will be necessary to enable real-time decision support (124).
Improved Kidney Disease Risk Assessment
The development of tools to improve KD risk assessment and prognosis may also be beneficial. Multiple risk assessment equations exist for different patient populations. The Kidney Failure Risk Equations (KFRE) are the most internationally validated, widely known, and widely used risk assessment equations for people with eGFR<60 mL/min/1.73 m2 (125). The KFREs can be used to predict an individual’s 2–5-year risk of developing kidney failure and were originally developed in Canadian patients diagnosed with stages G3–5 CKD (125, 126). The KFREs have been extensively validated in >700 000 individuals across >30 countries and demonstrated high discrimination between individuals who develop kidney failure and individuals who do not. Specifically, there are 2 KFREs, a 4-variable KFRE, and an 8-variable KFRE. The 4-variable KFRE derives kidney failure risk from an individual’s age, sex, eGFR, and uACR; the 8-variable equation includes the aforementioned parameters in addition to serum albumin, bicarbonate, calcium, and phosphorus measurements. Results are reported as percentage risk, ranging from <1% to 99.99%. The 4-variable KFRE was superior to 5 different eGFR equations (CKD-EPI 2009 eGFRcr, CKD-EPI-2021 eGFRcr, CKD-EPI 2012 eGFRcys, CKD-EPI 2012 eGFRcr-cys, and CKD-EPI 2021 eGFRcr-cys) at predicting 2-year risk for kidney failure (127). A KFRE score of >20% has sensitivities ranging from 0.68 to 0.78, as compared to 0.42 to 0.66 when using a common eGFR cutoff point of <20 mL/min/1.73 m2 across all eGFR equations assessed, for dialysis referral or kidney transplant recommendation. Of note, in patients diagnosed with autosomal dominant polycystic KD, the KFRE underestimated risk, while in elderly patients (80 years and older) the KFRE overestimated the risk of kidney failure (126, 128). Indications for calculating KFRE risk scores are not well-defined and continued validation of the equations will be necessary to define how often the risk score should be calculated.
Supplemental Material
Supplemental material is available at The Journal of Applied Laboratory Medicine online.
Nonstandard Abbreviations: KD, kidney disease; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; NKF, National Kidney Foundation; ASN, American Society of Nephrology; KDIGO, Kidney Disease Improving Global Outcomes; eGFRcr, estimated glomerular filtration rate calculated with creatinine; CKD, chronic kidney disease; eGFRcr-cys, estimated glomerular filtration rate calculated with creatinine and cystatin C; eGFRcys, estimated glomerular filtration rate calculated with cystatin C; SDI, social deprivation index; ESKD, end-stage kidney disease; mGFR, measured glomerular filtration rate; 4v-MDRD, 4-variable Modification of Diet in Renal Disease; uACR, urine albumin to creatinine ratio; AKI, acute kidney injury; LLOQ, lower limit of quantification; uPCR, urine protein to creatinine ratio; 6v-MDRD, 6-variable Modification of Diet in Renal Disease; KDOQI, Kidney Disease Outcomes Quality Initiative; MDRD, Modification of Diet in Renal Disease; EHR, electronic health record; LIS, laboratory information system; POC, point of care; USPSTF, United States Preventative Services Task Force; KPMP, Kidney Precision Medicine Project; KFRE, kidney failure risk equation; CMS, Centers for Medicare and Medicaid Services; CPT, Current Procedure Terminology.
Author Contributions: The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Christina Pierre (Conceptualization-Equal, Methodology-Equal, Project administration-Equal, Writing—original draft-Lead, Writing— review & editing-Lead), Mark Marzinke (Conceptualization-Equal, Methodology-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Sofia B. Ahmed (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), David Collister (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Jessica Colon-Franco (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Melanie Hoenig (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Thomas Lorey (Conceptualization-Supporting, Writing—original draft-Supporting, Writing—review & editing-Supporting), Paul M. Palevsky (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Octavia Peck Palmer (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Sylvia Rosas (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Joseph Vassalotti (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), Cameron T. Whitley (Conceptualization-Equal, Writing—original draft-Supporting, Writing—review & editing-Supporting), and Dina Greene (Conceptualization-Lead, Writing—original draft-Lead, Writing—review & editing-Lead).
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Research Funding: D. Collister, CIHR Project Grant 2023 GAHT-Kidney 2023-present, CIHR Project Grant 2022 RESET-DIALYSIS 2022-present, KFOC KRESCENT New Investigator Award 2020–2023, Research Manitoba grant Virtual Kidney Check and Follow-up 2021-present, Boehringer Ingelheim grant Virtual Kidney Check and Follow-up 2021-present, University of Alberta Startup Funds 2021-present. Disclosures: P.M. Palevsky is President, National Kidney Foundation, 2020–2022 and Immediate Past-President, National Kidney Foundation, 2022-present, and received consulting fees from Jannsen Research & Development, unrelated to the present work, and funding from the NIH/NIDDK (1U01 DK130010, 1UH3 DK114861, 1RO1DK128100, R21 DK122023), unrelated to the present work. T. Lorey is an Associate Editor for The Journal of Applied Laboratory Medicine, AACC. O.M. Peck Palmer received support from AACC, Cytovale, NIH, Roche Diagnostics, Siemens Healthineers, and Werfen and is on the AACC Board of Directors. S. Ahmed, Advisory Board Member, Canadian Institutes of Health Research Institute of Gender and Health, President-Elect, Organization for the Study of Sex Difference, Member, Canadian Medical Association Journal Governance Council. D. Collister, Scientific Chair of the Canadian Nephrology Trials Network, Member of the Canadian Society of Nephrology Scientific Committee, Accelerating Clinical Trials IDEA Committee and recipient of KFOC KRESCENT New Investigator Award 2020–2023. M. Hoenig, honoraria for speaking at PriMED (primary care conference) about CKD. M.A. Marzinke, consulting fees from Bio-Rad, royalties from Elsevier, grant funding from NIH, ViiV Healthcare, Gilead Sciences, Merck and Dohme, Ridgeback Therapeutics. S. Rosas, research funds to institution from Bayer and AstraZeneca, Scientific Advisory Boards of Bayer, Teladoc, and AstraZeneca, employee of Joslin Diabetes Center, Boston, MA, and President of National Kidney Foundation. J. Vassalotti, AstraZeneca, Inc.—consulting honoraria—diabetic kidney disease, Renalytix, plc—consulting honoraria—risk stratification in diabetic kidney disease, Chief Medical Officer of National Kidney Foundation and member of Renalytix, plc Advisory Board. C.C. Pierre has received travel support from AACC. D.N. Greene, Associate Editor for The Journal of Applied Laboratory Medicine, AACC, and member of scientific advisory for Abbott Diagnostics Point of Care division in relation to screening for CKD.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Acknowledgments: The authors acknowledge the constructive feedback on draft iterations of this manuscript by the AACC Academy and AACC members, especially Greg Miller Ph.D. We would also like to acknowledge the constructive feedback on draft iterations of this manuscript by the National Kidney Foundation’s Scientific Advisory Board, and Andrew S. Levey, MD. We appreciate the guidance of AACC leadership, Alison Woodworth PhD, Allison Chambliss PhD, and Yusheng Zhu PhD, and AACC staff member Stefanie Kleinman throughout the preparation of this document.
References
- US Department of Health and Human Services. Office of Minority Health. https://www.minorityhealth.hhs.gov/ (Accessed August 2022).
- Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call US to act. Lancet 2020;396:1125–8.
- Owens K, Walker A. Those designing healthcare algorithms must become actively anti-racist. Nat Med. NIH Public Access 2020;26:1327.
- Delgado C, Baweja M, Ríos Burrows N, Crews DC, Eneanya ND, Gadegbeku CA, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. JASN 2021;32:1305-17.
- Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis 2021;9:268–288.e1.
- Kattari SK, Walls NE, Whitfield DL, Langenderfer Magruder L. Racial and ethnic differences in experiences of discrimination in accessing social services among transgender/gender-nonconforming people. J Soc Work Disabil Rehabil 2016;26:217–35.
- CDC. Chronic kidney disease in the United States, 2021. Atlanta (GA): Centers for Disease Control and Prevention; 2021.
- International Society of Nephrology. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:136–50.
- United States Renal Data System. 2022 USRDS Annual data report: Epidemiology of kidney disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2022.
- Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, et al. Sex-related disparities in CKD progression. J Am Soc Nephrol 2019;30:137–46.
- Albertus P, Morgenstern H, Robinson B, Saran R. Risk of ESRD in the United States. Am J Kidney Dis 2016;68:862–72.
- De La Mata NL, Rosales B, Macleod G, Kelly PJ, Masson P, Morton RL, et al. Sex differences in mortality among binational cohort of people with chronic kidney disease: population based data linkage study. BMJ 2021;375: e068247.
- Evans K, Coresh J, Bash LD, Gary-Webb T, Köttgen A, Carson K, et al. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant 2011;26:899.
- United States Renal Data System. 2021 USRDS Annual data report: Epidemiology of kidney disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021.
- Crews DC, Banerjee T, Wesson DE, Morgenstern H, Saran R, Burrows NR, et al. Race/ethnicity, dietary acid load, and risk of end-stage renal disease among US adults with chronic kidney disease. Am J Nephrol 2018;47:174–81.
- Kramer H. Diet and chronic kidney disease. Adv Nutr 2019;10:S367–79.
- Regele F, Jelencsics K, Shiffman D, Paré G, McQueen MJ, Mann JFE, et al. Genome-wide studies to identify risk factors for kidney disease with a focus on patients with diabetes. Nephrol Dial Transplant. 2015;30:iv26–34.
- Cañadas-Garre M, Anderson K, Cappa R, Skelly R, Smyth LJ, McKnight AJ, et al. Genetic susceptibility to chronic kidney disease—some more pieces for the heritability puzzle. Front Genet 2019;10:453.
- Divers J, Freedman BI. Susceptibility genes in common complex kidney disease. Curr Opin Nephrol Hypertens 2010;19:79.
- Robert Graham Center. Social Deprivation Index (SDI).
https://www.graham-center.org/maps-data-tools/ social-deprivation-index.html (Accessed April 2022).
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the National Kidney Disease Education Program. Clin Chem 2006;52:5–18.
- Friedman DJ, Pollak MR. APOL1 nephropathy: from
genetics to clinical applications. Clin J Am Soc Nephrol 2021;16:294–303.
- Miller WG, Kaufman HW, Levey AS, Straseski JA, Wilhelms KW, Yu H-Y, et al. National kidney foundation laboratory engagement working group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clin Chem 2022;68: 511–20.
- Zhang X, Rule AD, McCulloch CE, Lieske JC, Ku E, Hsu CY. Tubular secretion of creatinine and kidney function: an observational study. BMC Nephrol 2020;21:1–9.
- Lemoine S, Panaye M, Pelletier C, Bon C, Juillard L, Dubourg L, et al. Cystatin C-creatinine based glomerular filtration rate equation in obese chronic kidney disease patients: impact of deindexation and gender. Am J Nephrol 2016;44:63–70.
- Kervella D, Lemoine S, Sens F, Dubourg L, Sebbag L, Guebre-Egziabher F, et al. Cystatin C versus creatinine for GFR estimation in CKD due to heart failure. Am J Kidney Dis 2017;69:321–3.
- Torre A, Aguirre-Valadez JM, Arreola-Guerra JM, García-Flores OR, García-Juárez I, Cruz-Rivera C, et al. Creatinine versus cystatin C for estimating GFR in patients with liver cirrhosis. Am J Kidney Dis 2016;67: 342–4.
- Krones E, Fickert P, Zitta S, Neunherz S, Artinger K, Reibnegger G, et al. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol 2015;16:196.
- Teaford HR, Barreto JN, Vollmer KJ, Rule AD, Barreto EF, Kern PE. Cystatin C: a primer for pharmacists. Pharmacy 2020;8:35.
- Liu Y, Xia P, Cao W, Liu Z, Ma J, Zheng K, et al. Divergence between serum creatine and cystatin C in estimating glomerular filtration rate of critically ill COVID-19 patients. Ren Fail 2021;43:1104–14.
- Ye Y, Gai X, Xie H, Jiao L, Zhang S. Impact of thyroid function on serum cystatin C and estimated glomerular filtration rate: a cross-sectional study. Endocr Pract 2013;19:397–403.
- Xin C, Xie J, Fan H, Sun X, Shi B. Association between serum cystatin C and thyroid diseases: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12:766516.
- The Kidney Precision Medicine Project. KPMP [Home page]. https://www.kpmp.org/ (Accessed August 2022).
- Chen DC, Potok OA, Rifkin DE, Estrella MM. Advantages, limitations, and clinical considerations in using cystatin C to estimate GFR. Kidney360 2022;3:1807–14.
- Knight EL, Verhave JC, Spiegelman D, Hillege HL, De Zeeuw D, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004; 65:1416–21.
- Shlipak MG, Inker LA, Coresh J. Serum cystatin C for estimation of GFR. JAMA 2022;328:883–4.
- Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, et al. Factors other than GFR affecting serum cystatin C levels. Kidney Int 2009;75:652.
- Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function—a review. Clin Chem Lab Med 1999;37:389–95.
- Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49.
- Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–9.
- El-Khoury JM, Hoenig MP, Jones GRD, Lamb EJ, Parikh CR, Tolan NV, et al. AACC Guidance document on laboratory investigation of acute kidney injury. J Appl Lab Med 2021; 6:1316–37.
- Teaford HR, Rule AD, Mara KC, Kashani KB, Lieske JC, Schreier DJ, et al. Patterns of cystatin C uptake and use across and within hospitals. Mayo Clin Proc 2020;95: 1649–59.
- Comper WD, Vuchkova J, McCarthy KJ. New insights into proteinuria/albuminuria. Front Physiol 2022;13:1–16.
- Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35.
- Seegmiller JC, Miller WG, Bachmann LM. Moving toward standardization of urine albumin measurements. EJIFCC 2017;28:258.
- Miller WG, Seegmiller JC, Lieske JC, Narva AS, Bachmann LM. Standardization of urine albumin measurements: status and performance goals. J Appl Lab Med 2017;2: 423–9.
- Bachmann LM, Nilsson G, Bruns DE, McQueen MJ, Lieske JC, Zakowski JJ, et al. State of the art for measurement of urine albumin: comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin Chem. Oxford Academic 2014;60:471–80.
- Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Yuan HC, Feldman HI, et al. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis 2018;72:538.
- Miller WG, Bachmann LM, Fleming JK, Delanghe JR, Parsa A, Narva AS. Recommendations for reporting low and high values for urine albumin and total protein. Clin Chem 2019;65:349–50.
- Greene DN, Marzinke MA, Carter C, Chen J, Hoenig MP, Rummel M. Decreasing the lower limit of quantitation for urine albumin improves clinical utility. J Appl Lab Med 2022;7:1145–50.
- Miller MW G, Bachmann LM, Delanghe JR, Inker LA, Jones GRD, Vassalotti JA. Optimal use of biomarkers for chronic kidney disease. Clin Chem 2019;65:949–55.
- Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. Conversion of urine protein–creatinine ratio or urine dipstick protein to urine albumin– creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant– based meta-analysis. Ann Intern Med 2020;173: 426–35.
- National Kidney Foundation Laboratory Engagement Advisory Group. Laboratory engagement plan: Transforming kidney disease detection. New York (NY): National Kidney Foundation; 2018.
- College of American Pathologists. Educational discussion: 2020-A chemistry survey (C). Kidney biomarkers: the kidney profile order, urine albumin- creatinine ratio (uACR), and estimated glomerular filtration rate (eGFR). Northfield (IL): College of American Pathologists; 2020.
- Shin JI, Chang AR, Grams ME, Coresh J, Ballew SH, Surapaneni A, et al. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension 2021;78:1042–52.
- Stempniewicz N, Vassalotti JA, Cuddeback JK, Ciemins E, Storfer-Isser A, Sang Y, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care 2021;44:2000–9.
- Alfego D, Ennis J, Gillespie B, Lewis MJ, Montgomery E, Ferrè S, et al. Chronic kidney disease testing among at-risk adults in the U.S. remains low: real-world
evidence from a national laboratory database. Diabetes Care 2021;44:2025.
- Vassalotti JA, Boucree SC. Integrating CKD into US primary care: bridging the knowledge and implementation gaps. Kidney Int Rep 2022;7:389–96.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. Nephron 1976;16:31–41.
- Hudson JQ, Nolin TD. Pragmatic use of kidney function estimates for drug dosing: the tide is turning. Adv Chronic Kidney Dis 2018;25:14–20.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–70.
- Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med 2021;384:474–80.
- Jorde LB, Wooding SP. Genetic variation, classification and “race”. Nat Genet 2004;36:S28–33.
- Micheletti SJ, Bryc K, Ancona Esselmann SG, Freyman WA, Moreno ME, Poznik GD, et al. Genetic consequences of the transatlantic slave trade in the Americas. Am J Hum Genet 2020;107:265–77.
- Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet 2015;96:37–53.
- Olufadeji A, Dubosh NM, Landry A. Guidelines on the use of race as patient identifiers in clinical presentations. J Natl Med Assoc 2021;113:428–30.
- Eneanya ND, Boulware LE, Tsai J, Bruce MA, Ford CL, Harris C, et al. Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol 2021;18: 84–94.
- Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020;383:874–82.
- Hoenig MP, Mann A, Pavlakis M. Removal of the black race coefficient from the estimated glomerular filtration equation improves transplant eligibility for black patients at a single center. Clin Transplant 2022;36:e14467.
- Zelnick LR, Leca N, Young B, Bansal N. Association of the estimated glomerular filtration rate with vs without a coefficient for race with time to eligibility for kidney transplant. JAMA Netw Open 2021;4:e2034004.
- Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, et al. Effect of genetic African ancestry on eGFR and kidney disease. J Am Soc Nephrol 2015;26: 1682–92.
- Peralta CA, Risch N, Lin F, Shlipak MG, Reiner A, Ziv E, et al. The association of African ancestry and elevated creatinine in the coronary artery risk development in young adults (CARDIA) study. Am J Nephrol 2010;31:202–8.
- Marzinke MA, Greene DN, Bossuyt PM, Chambliss AB, Cirrincione LR, McCudden CR, et al. Limited evidence for use of a black race modifier in eGFR calculations: a systematic review. Clin Chem 2021;68:521–33.
- U.S. Census Bureau. QuickFacts: United States. https://www.census.gov/quickfacts/fact/table/US/PST045221 (Accessed December 2022).
- Chu CD, McCulloch CE, Banerjee T, Pavkov ME, Burrows NR, Gillespie BW, et al. CKD Awareness among US adults by future risk of kidney failure. Am J Kidney Dis 2020;76: 174–83.
- National Kidney Foundation. CKDintercept. https://www.kidney.org/CKDintercept (Accessed April 2022).
- National Kidney Foundation. Leading the way to advance early diagnosis of chronic kidney disease. https://www. kidney.org/CKDintercept/laboratoryengagement (Accessed April 2022).
- Mendu ML, Tummalapalli SL, Lentine KL, Erickson KF, Lew SQ, Liu F, et al. Measuring quality in kidney care: an evaluation of existing quality metrics and approach to facilitating improvements in care delivery. J Am Soc Nephrol 2020;31:602–14.
- Renal Physicians Association. Physician Performance Measurement. https://www.renalmd.org/page/ physiciandevelopment (Accessed April 2022).
- National Quality Forum. Renal Spring 2018–Fall 2018 Cycle: CDP Report [Technical Report]. Washington (DC): National Quality Forum; 2019.
- National Committee for Quality Assurance. HEDIS measures and technical resources. https://www.ncqa. org/hedis/measures/ (Accessed April 2022).
- Quality Payment Program. Explore Measures & Activities. https://qpp.cms.gov/mips/explore-measures (Accessed April 2022).
- Centers for Medicare & Medicaid Services. ESRD quality incentive program. https://www.cms.gov/medicare/ quality-initiatives-patient-assessment-instruments/ esrdqip (Accessed April 2022).
- Committee ADAPP. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care 2022;45:S175–84.
- Erez DL, Derwick H, Furth S, Ballester L, Omuemu S, Adiri Y, et al. Dipping at home: is it better, easier, and more convenient? A feasibility and acceptability study of a novel home urinalysis using a smartphone application. Pediatr Nephrol 2023;38:139–43.
- Leddy J, Green JA, Yule C, Molecavage J, Coresh J, Chang AR. Improving proteinuria screening with mailed smartphone urinalysis testing in previously unscreened patients with hypertension: a randomized controlled trial. BMC Nephrol 2019;20:1–7.
- Burke AE, Thaler KM, Geva M, Adiri Y. Feasibility and acceptability of home use of a smartphone-based urine testing application among women in prenatal care. Am J Obstet Gynecol. Mosby 2019;221:527–8.
- Jurkovitz CT, Li S, Norris KC, Saab G, Bomback AS, Whaley-Connell AT, et al. Association between lack of health insurance and risk of death and ESRD: results from the kidney early evaluation program (KEEP). Am J Kidney Dis 2013;61:S24.
- Vassalotti JA, DeVinney R, Lukasik S, McNaney S, Montgomery E, Voss C, et al. CKD Quality improvement intervention with PCMH integration: health plan results. Am J Manag Care 2019;25:294–301.
- Park KJ, Unitan RS, Thorp ML. A quality improvement initiative targeting chronic kidney disease metrics through increased urinary albumin testing. Perm J 2020; 25:210.
- Silver SA, Bell CM, Chertow GM, Shah PS, Shojania K, Wald R, et al. Effectiveness of quality improvement strategies for the management of CKD: a meta-analysis. Clin J Am Soc Nephrol 2017;12:1601–14.
- Sim JJ, Rutkowski MP, Selevan DC, Batech M, Timmins R, Slezak JM, et al. Kaiser Permanente creatinine safety program: a mechanism to ensure widespread detection and care for chronic kidney disease. Am J Med 2015; 128:1204–1211.e1.
- Sequist T, Holliday A, Orav J, Bates D, Denker B. Physician and patient tools to improve chronic kidney disease care. Am J Manag Care 2018;24:e107–14.
- Tuot DS, McCulloch CE, Velasquez A, Schillinger D, Yuan HC, Handley M, et al. Impact of a primary care CKD registry in a US public safety-net health care delivery system: a pragmatic randomized trial. Am J Kidney Dis 2018;72:168–77.
- National Kidney Foundation. Recommendations for implementing the CKD-EPI 2021 race-free eGFR calculation: Guidelines for clinical laboratories. https:// www.kidney.org/content/national-kidney-foundation- laboratory-engagement-working-group- recommendations-implementing (Accessed May 2022).
- National Kidney Foundation. eGFR Calculator. https://www.kidney.org/professionals/kdoqi/gfr_calculator (Accessed May 2022).
- Toffaletti JG, Burke CO, Bayliss G, Lynch M. Utilizing longitudinal within-individual changes of serum creatinine, cystatin C, and/or eGFR to optimize clinical sensitivity and eliminate race and gender corrections. J Appl Lab Med 2022;7:807–11.
- Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M, Sequist TD. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med 2016;129:153–162.e7.
- Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H. Article A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 2015;10: 382–9.
- Shafi T, Zhu X, Lirette ST, Rule AD, Mosley T, Butler KR, et al. Quantifying individual-level inaccuracy in glomerular filtration rate estimation: a cross-sectional study. Ann Intern Med 2022;175:1073–82.
- Hilderink JM, Van Der Linden N, Kimenai DM, Litjens EJR, Klinkenberg LJJ, Aref BM, et al. Biological variation of creatinine, cystatin C, and eGFR over 24 hours. Clin Chem 2018;64:851–60.
- LOINC. Choosing the Correct LOINC for Estimated Glomerular Filtration Rate. https://loinc.org/kb/users- guide/loinc-technical-briefs/choosing-the-correct-loinc- for-estimated-glomerular-filtration-rate/ (Accessed August 2022).
- Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol 2020;15:1203–12.
- Walther CP, Winkelmayer WC, Navaneethan SD. Updated US prevalence estimates for chronic kidney disease stage and complications using the new race-free equation to estimate glomerular filtration rate. JAMA Netw Open 2022;5:e220460.
- Akhimiona CO, Nguyen DT, Graviss EA, Gaber AO, Suki WN. Suitability of estimated glomerular filtration rate for live kidney donor selection. Transplant Proc 2018;50:3071–5.
- Organ Procurement and Transplantation Network. OPTN Board of Directors expected to require transplant hospitals to use race-neutral calculations in assessing patients. https://optn.transplant.hrsa.gov/news/optn- board-of-directors-expected-to-require-transplant- hospitals-to-use-race-neutral-calculations-in-assessing- patients/ (Accessed August 2022).
- US Food & Drug Administration. Pharmacokinetics in patients with impaired renal function–Study design, data analysis, and impact on dosing and labeling. https:// www.fda.gov/regulatory-information/search-fda- guidance-documents/pharmacokinetics-patients- impaired-renal-function-study-design-data-analysis- and-impact-dosing-and (Accessed May 2022).
- Ebert N, Shlipak MG. Cystatin C is ready for clinical use. Curr Opin Nephrol Hypertens 2020;29:591–8.
- Roland Markos JI, Schaepe KS, Teaford HR, Rule AD, KashaniID KB, LieskeID JC, et al. Clinician perspectives on inpatient cystatin C utilization: a qualitative case study at mayo clinic. PLoS ONE 2020;15:e0243618.
- Barreto EF, Rule AD, Murad MH, Kashani KB, Lieske JC, Erwin PJ, et al. Prediction of the renal elimination of drugs with cystatin C vs creatinine: a systematic review. Mayo Clin Proc 2019;94:500–14.
- Chen DC, Shlipak MG, Scherzer R, Bauer SR, Potok OA, Rifkin DE, et al. Association of intraindividual difference in estimated glomerular filtration rate by creatinine vs cystatin C and end-stage kidney disease and mortality. JAMA Netw Open 2022;5:e2148940.
- Fu E, Levey A, Coresh J, Elinder C, Rotmans J, Dekker F, et al. Accuracy of GFR estimating equations in patients with discordances between creatinine and cystatin C- based estimations. [Epub ahead of print] J Am Soc Nephrol March 30, 2023 as doi: 10.1681/ASN. 0000000000000128.
- Nadolsky KZ. Cystatin C, diabetic kidney disease, and implications for diabetes management. Endocr Pract 2017;23:241–2.
- Shardlow A, Mcintyre NJ, Fraser SDS, Roderick P, Raftery J, Fluck RJ, et al. The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: a primary care cohort study. PLoS Med 2017;14:e1002400.
- Centers for Medicare & Medicaid Services. 22CLABQ2. https://www.cms.gov/medicaremedicare-fee-service- paymentclinicallabfeeschedclinical-laboratory-fee- schedule-files/22clabq2 (Accessed April 2022).
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017;102:3869–903.
- Krupka E, Curtis S, Ferguson T, Whitlock R, Askin N, Millar AC, et al. The effect of gender-affirming hormone therapy on measures of kidney function. Clin J Am Soc Nephrol 2022;17:1305–15.
- Greene DN, Dy GW, Osbun N, Whitley CT. Reply to “Kidney transplantation and donation in the transgender population: a single-institution case series.” Am J Transplant 2020;20:3693–4.
- Patel K, Lyon ME, Luu HS. Providing inclusive care for transgender patients: capturing sex and gender in the electronic medical record. J Appl Lab Med 2021;6:210–8.
- Legrand H, Werner K, Christensson A, Pihlsgård M, Elmståhl S. Prevalence and determinants of differences in cystatin C and creatinine-based estimated glomerular filtration rate in community-dwelling older adults: a cross-sectional study. BMC Nephrol 2017;18:350.
- United States Preventive Services Taskforce. Final recommendation statement: Chronic kidney disease: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/ chronic-kidney-disease-ckd-screening (Accessed August 2022).
- Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Feature kidney disease, race, and GFR estimation. CJASN 2020; 15:1203–12.
- Sitapati A, Kim H, Berkovich B, Marmor R, Singh S, El-Kareh R, et al. Integrated precision medicine: the role of electronic health records in delivering personalized treatment. Wiley Interdiscip Rev Syst Biol Med 2017;9:1378.
- Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305: 1553–9.
- Hundemer GL, Tangri N, Sood MM, Clark EG, Canney M, Edwards C, et al. The effect of age on performance of the kidney failure risk equation in advanced CKD. Kidney Int Reports 2021;6:2993–3001.
- Bundy JD, Mills KT, Anderson AH, Yang W, Chen J, He J. Prediction of end-stage kidney disease using estimated glomerular filtration rate with and without race. Ann Intern Med 2022;175:305–13.
- Hundemer GL, Tangri N, Sood MM, Ramsay T, Bugeja A, Brown PA, et al. Performance of the kidney failure risk equation by disease etiology in advanced CKD. Clin J Am Soc Nephrol 2020;15:1424–32.