Words of Caution on the CMS Meaningful Use Standards
An Interview with Thomas Williams, MD, and Alexis Carter, MD

 Electronic health records (EHRs) parovide many benefits for providers and their patients, but the benefits depend on how well EHRs are implemented and used. The Center for Medicare and Medicaid Services (CMS) has defined a set of standards, commonly known as "meaningful use," that allow eligible providers and hospitals to earn incentive payments if their EHRs meet specific criteria. While the goal of meaningful use is to promote the spread of EHRs and to improve healthcare in the U.S., changes in EHRs are occurring so rapidly that laboratories may not even be aware of potential patient safety dangers.
Electronic health records (EHRs) parovide many benefits for providers and their patients, but the benefits depend on how well EHRs are implemented and used. The Center for Medicare and Medicaid Services (CMS) has defined a set of standards, commonly known as "meaningful use," that allow eligible providers and hospitals to earn incentive payments if their EHRs meet specific criteria. While the goal of meaningful use is to promote the spread of EHRs and to improve healthcare in the U.S., changes in EHRs are occurring so rapidly that laboratories may not even be aware of potential patient safety dangers.
In this interview, Thomas Williams, MD, and Alexis Carter, MD, provide their perspectives on meaningful use and offer suggestions to manage requests for laboratory data outside of official laboratory reports. Williams is medical director of pathology at Methodist Hospital in Omaha, Neb., while Carter is director of pathology informatics in the department of pathology and laboratory medicine at Emory University School of Medicine in Atlanta. Both pathologists, they served as members of a team organized by the Office of the National Coordinator for Health Information Technology, the U.S. government office responsible for establishing guidelines for meaningful use.
Corinne R. Fantz, PhD, DABCC, FACB conducted this interview.
Can you explain the difference between "official" and "unofficial" laboratory reports?
Williams: CLIA Interpretive Guidelines define fulfillment of the laboratory's official reporting responsibility as an event marked by delivery of the result to the authorized person (1). Additionally, CLIA requires that official laboratory reports contain certain elements such as reference ranges and units of measure. However, the CLIA regulations were created in the era of paper charts. With the advent of EHRs, less conservative CLIA interpretations have emerged, limiting the official laboratory report to a particular view or views in the EHR. EHR vendors have come under pressure to create alternate, abridged views displaying large amounts of patient data on relatively small screens. These views may constitute an unofficial report. Although the primary aim is to promote rapid and accurate patient assessments, ironically, poorly designed displays may jeopardize patient safety.
What are the most common reasons a physician would want to view laboratory results or abstract laboratory data outside of the official lab report?
Carter: Speed, efficiency, and increased ability to comply with disease-specific clinical quality measurements (CQM), such as for diabetes and hypertension, are commonly cited reasons. Clinicians will note that they do this now by writing or typing abbreviated laboratory data into the patient's medical record or using a disease-specific form to record data in a way that helps them quickly analyze it for more efficient patient care. We are fairly early in the use of these abstract views of laboratory data, so their effectiveness to accomplish the above goals has yet to be proven. We need studies on the potential risks associated with abbreviated laboratory data to understand the degree to which unexpected laboratory results might be missed because they are not part of the display.
How has meaningful use changed the way physicians interact with the EHR?
Carter: Meaningful use is the common term for a set of standards and CQM that emerged from the American Recovery and Reinvestment Act (ARRA), more specifically from the Health Information Technology for Economic and Clinical Health (HITECH) Act that is embedded within ARRA. Meaningful use standards and HITECH have the goal of modernizing the American health information technology (IT) infrastructure in a useful and meaningful manner. EHRs were chosen as the primary vehicle by which these goals would be achieved (2). There are substantial monetary rewards for timely adoption, defined as meeting specified functionality or criteria. In addition, those who cannot meet the minimum requirements will be penalized by reduced reimbursement from Medicare. To prove compliance with meaningful use, covered entities and eligible providers must demonstrate use of a certified EHR in a meaningful manner by attesting to fulfillment of certain criteria: by electronically exchanging health information to improve quality of healthcare; and by submitting clinical quality and other data from the EHR to the federal government.
Relatively few meaningful use criteria affect how laboratory data are displayed and used, and most are non-specific. For example, one objective requires that laboratory data be included in an EHR as structured data, but it does not specify to what degree the data should be structured. This means that rendering laboratory data as free text reports would technically be acceptable but arguably not acceptable to many busy providers who have to wade through them. Other objectives that affect laboratories include using computerized provider order entry (CPOE) for laboratory tests and requiring delivery of laboratory results to patients by electronic means such as patient portals.
Proper display and formatting of laboratory data can be a challenge, even for laboratories. What are the patient safety concerns with moving the lab data to an unofficial report?
Williams: The patient safety concerns are the potential for misinterpreting and missing laboratory data. Considerable literature exists regarding safe data formatting for legibility, such as font size, spacing, and density (3). Similarly, The Institute for Safe Medication Practices and The Joint Commission both have lists of abbreviations labeled "Do Not Use" because of their potential for medication-related error and patient harm (4). Display-related errors vary from aligning laboratory results from different dates into a single column, which may give the provider a misleading view of the sequence of health-related events, to marking abnormal laboratory results with a flag that closely resembles a number, which may cause the provider to misinterpret the numerical laboratory result and treat the patient incorrectly. Other errors have included missing mycobacterial culture results, because the results were sequenced by date of collection. This would cause the results to be displayed 4 weeks after the collection, far outside the normal view of laboratory data.
Examples of Unofficial Lab Reports
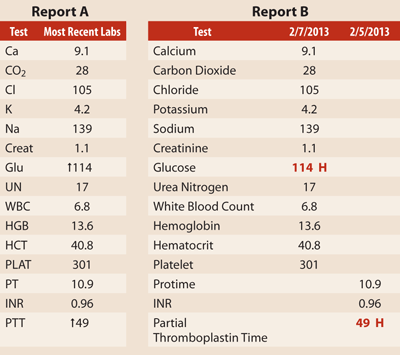 Both of these laboratory reports lack all of the elements required by CLIA; therefore, they would be considered unofficial laboratory reports. Neither report has units of measure or reference ranges; however, Report A is a greater threat to patient safety. In this report, the patient's glucose and PTT are elevated. Providers could erroneously interpret the arrows indicating the elevation as numerals, leading to a finding of extreme elevations for these values. It is also not clear if the patient results are from the same date and time, as Report A only provides the "most recent labs." Report B has some of the same problems as Report A, but it does indicate the dates the tests were performed and draws attention to abnormally high results with the letter "H" and red numbers. However, the report has no space for comments such as a hemolyzed specimen and does not indicate whether the results of other tests are still pending.
Both of these laboratory reports lack all of the elements required by CLIA; therefore, they would be considered unofficial laboratory reports. Neither report has units of measure or reference ranges; however, Report A is a greater threat to patient safety. In this report, the patient's glucose and PTT are elevated. Providers could erroneously interpret the arrows indicating the elevation as numerals, leading to a finding of extreme elevations for these values. It is also not clear if the patient results are from the same date and time, as Report A only provides the "most recent labs." Report B has some of the same problems as Report A, but it does indicate the dates the tests were performed and draws attention to abnormally high results with the letter "H" and red numbers. However, the report has no space for comments such as a hemolyzed specimen and does not indicate whether the results of other tests are still pending.
CLIA covers the requirement for laboratory result reporting. Are there any requirements for unofficial laboratory report displays?
Carter: Although there are no formal requirements for laboratory results outside of what is required for official laboratory reports by CLIA and the College of American Pathologists, healthcare entities and laboratories should use care in how laboratory data are displayed. Formats that substantially depart from what would be considered usual and customary should be examined for the potential to cause harm before implementing them.
What role and responsibility does an EHR vendor have in generating unofficial lab reports?
Williams: Vendors usually develop these views at the request of providers; therefore, the vendor certainly has a role in their construction. However, the provider is ultimately responsible for patient care. As such, these views may constitute a significant and underappreciated medical risk to patients and a medico-legal risk to clinicians, local IT providers, EHR vendors, and laboratory medical directors. The risk is even greater when laboratorians do not review displays of the data that their laboratory generates. This is not uncommon, especially with patient portals that may import laboratory data from the EHR rather than the laboratory information system. When CLIA-required elements are not present, which would constitute an unofficial view, or when other problems occur, such as missing data or incorrectly displayed data, the question of who will be legally and medically responsible following an untoward event is untried to date.
Who should be notified when an unofficial or unapproved display of laboratory data is requested in an EHR?
Carter: Ideally, the laboratory director and/or laboratory informatics director should partner with their institution's health IT leadership, such as the chief medical information officer or chief information officer, to encourage a comprehensive review of EHR displays of laboratory data by a qualified laboratorian. Proactive on-going efforts to improve laboratory displays should be made with teams, including laboratory directors, providers, health IT staff, and patients. When unofficial displays are discovered that have not been through a formal review process, any potential risks to patients or providers should be addressed with the above teams.
Do you have suggestions about how labs can contribute to building safer unofficial laboratory reports?
Williams: Spurred by the introduction of meaningful use, the development of novel and technically unofficial laboratory reports is now outpacing legal and regulatory guidelines. Notably, these displays of laboratory data are often used outside the oversight of laboratory professionals. Therefore, it is critical that laboratory directors be proactive in their approach by being involved, asking questions, and sharing their views and expertise (See Box, below).

REFERENCES
- Centers for Disease Control and Prevention. Current CLIA regulations. §493.1291 Standard: Test report. Available online. (Accessed March 2013).
- Centers for Disease Control and Prevention. Meaningful use. Available online. (Accessed March 2013).
- University of Michigan. Legibility index for examining common viewing situations: A new definition using solid angle. Available online. (Accessed March 2013).
- Institute for Safe Medication Practices. Special issue—Do not use these dangerous abbreviations or dose designations. Available online. (Accessed March 2013).
OTHER RESOURCES
HHS Office of the National Coordinator (ONC) for Health Information Technology: Available online.
HHS ONC Meaningful Use: Available online.
Final Rule July 28, 2010 (276 pages): Available online.
Meaningful Use Stage 1 Grid: Available online.
Health Information Technology for Economic and Clinical Health (HITECH) Act
Title XIII of Division A and Title IV of Division B of the American Recovery and Reinvestment Act of 2009 (ARRA), Pub. L. No. 111-5, 123 Stat. 226 (Feb. 17, 2009), codified at 42 U.S.C. §§300jj et seq.; §§17901 et seq.
 Corinne Fantz, PhD, DABCC, FACB, is an associate professor of Pathology in the Department of Pathology and Labo-ratory Medicine at Emory University, Atlanta. She is also a member of the Patient Safety Editorial Board.
Corinne Fantz, PhD, DABCC, FACB, is an associate professor of Pathology in the Department of Pathology and Labo-ratory Medicine at Emory University, Atlanta. She is also a member of the Patient Safety Editorial Board.
Email: [email protected].