Introducing SYCL SnapShots
Welcome to SYCL SnapShots, a new quarterly column in CLN describing essential skills and practices for effective laboratory management. Here, members of AACC's Society for Young Clinical Laboratorians (SYCL) will present a snapshot of a topic, such as business basics, data analysis and statistics, or online tools. We hope CLN readers will find this new column valuable and we welcome your feedback.
 Christopher McCudden, PhD
Christopher McCudden, PhD
SYCL Chair
The Ottawa Hospital
Ottawa, Ontario, Canada
Email: [email protected]
Practical Considerations for Implementing a New Lab Analyzer
By Julie Shaw, PhD
As clinical chemists, we play a major role in evaluating and implementing new methods and analyzers. During fellowship training, we become well-versed in the method evaluation process, mainly from a theoretical point of view. Written guidelines, such as those published by the Clinical and Laboratory Standards Institute (CLSI), describe the technical components of a method evaluation in detail, including imprecision, method comparison, linearity, analytical sensitivity, carry-over and verification of reference intervals. But what happens after the decision has been made to purchase a new analyzer? This process is much less well-defined and lacks written guidelines. Furthermore, trainees may not get much exposure to this more practical side of laboratory operations. The flow chart presented here outlines these less well-documented steps, from the time the decision is made to purchase an analyzer until the beginning of patient testing.
Going Step-by-Step
First, a team must champion the implementation. Ideally, members would include laboratory bench, senior technical, and laboratory information system (LIS) staff. The team should assume primary responsibility for managing the project, including communicating with other departments within the hospital, as well as with external contacts, to facilitate a smooth transition. The team also should plan both a realistic and ideal "go-live" date for implementation, taking into account the needs of facilities management, information technology (IT), and laboratory operations.
Each stakeholder has specific needs that the team should take into account. Facilities management needs to be advised of the physical and environmental requirements for the new instrument. Changes may be required to the existing laboratory infrastructure, such as electrical and water. Larger-scale renovations may even be necessary. How the instrument will be delivered is a consideration, too. For example, is the service elevator large enough to accommodate the analyzer?
The IT department needs to be advised of changes required for specific tests that are being moved to the new analyzer and of any new tests being added to the existing menu. For example, the following would affect the LIS: changes to reference interval values, cut off values, critical values, and interpretative comments. These changes also will influence how results are reported in electronic medical records, so it is important to communicate and collaborate with the staff involved in making these changes.
Within the laboratory, consideration should be given to whether additional staff will be needed, especially if the instrument adds completely new tests to the lab's menu. Staff running the new analyzer also will require comprehensive, hands-on training, either on-site or off-site. Off-site training adds time and expense to the project.
Another consideration is test volume. The team should estimate the new work-load so that sufficient reagents can be ordered, as well as check on whether licensing is needed for any new tests.
Practical Steps for Implementing a New Analyzer
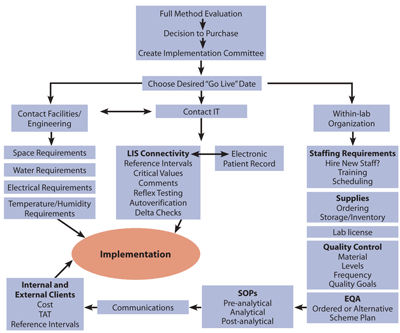 After the decision is made to purchase a new analyzer, a team should map out a plan for bringing it online in the lab.
After the decision is made to purchase a new analyzer, a team should map out a plan for bringing it online in the lab.
Planning for QC and PT
Another step is setting up appropriate quality control (QC) procedures. The team should decide which QC material the lab will use and set quality goals for each method. These decisions should be based on the method evaluation data, clinical need, and published requirements, such as those defined by Clinical Laboratory Improvement Amendments (CLIA) or other national programs.
An external quality assessment (EQA) or proficiency testing (PT) program is required for each test performed in the laboratory. The team should notify PT providers of any changes in methodology and request PT surveys for new tests. For some analytes, PT is not available and an alternative scheme must be developed, such as inter-lab comparison. As part of total quality management within the laboratory, the team should prepare standard operating procedures for each new pre-analytical, analytical, and post-analytical process associated with the new analyzer and tests. If an analyte will be measured on more than one instrument, inter-instrument comparisons also will be required.
Communicate!
Finally, prior to the go-live date, laboratory staff needs to communicate the impending changes to both internal and external clients, including the date on which the changes will occur. Communications should be planned well in advance so that there is ample time for client education about changes to reference ranges, cut off values, turnaround time, and new interpretive comments.
Celebrate Success
When the analyzer and new tests are up and running, it is always a good idea to review what went well and what didn't. And don't forget to thank committee members for all their hard work!

Julie Shaw, PhD, is a clinical biochemist at The Ottawa Hospital in Ottawa, Ontario, Canada and an assistant professor in the Department of Pathology and Laboratory Medicine at the University of Ottawa. Email: [email protected].
More information on AACC's SYCL is available online.