Case: A lean, nondiabetic 39 year old man presented to an outside hospital with severe upper abdominal pain. He had a history of alcoholism but he had no history of gall stones. He was diagnosed with acute pancreatitis, made npo and was treated with total parenteral nutrition (TPN).
Two days later he was transferred to the first author's hospital. At that time the patient's serum amylase was 787 U/L (reference interval: <60 U/L) and his serum lipase was 3850 U/L (reference interval: <100 U/L ). An abdominal CT scan was consistent with the diagnosis of acute pancreatitis.
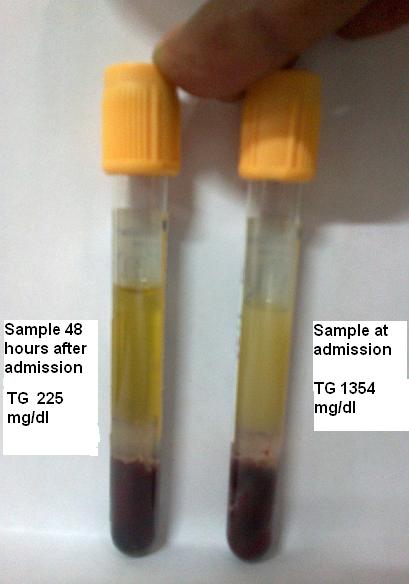
Because the serum was mildly lipemic, the next morning a serum triglyceride (TG) measurement was performed on the admission serum sample. The TG concentration was found to be greatly elevated at 1354 mg/dL (desirable range: <150 mg/dL).
What is the clinico-biochemical interpretation of such an elevated TG in the setting of acute pancreatitis?
Following alcohol-induced and gallstone-induced acute pancreatitis, the next most common cause of acute pancreatitis is hypertriglyceridemia that accounts for 1 – 4% of cases (1). Because of the patient's turbid serum and the recognized causal relationship of hypertriglyceridemia to acute pancreatitis, a TG measurement was recommended. Indeed the subsequent finding of a markedly elevated TG level suggested hypertriglyceridemia as a significant contributor to the development of patient's acute pancreatitis. However, this is not the end of this story.
In fact, this is only the beginning of the story.
While interpreting laboratory results in acute pancreatitis, it is important to recall that total parenteral nutrition (TPN) may be used in the management of acute pancreatitis (2). For adults, standard TPN formulations contain amino acids, dextrose, a fat emulsion, electrolytes, trace elements, vitamins and glycerol. Enteral nutrition (EN) may also be used in the treatment of acute pancreatitis.
Triglyceride measurements performed on automated chemistry analyzers can be of two types: glycerol-blanked and non-glycerol-blanked. Most laboratories (including the author's laboratories) use non-glycerol-blanked methods. Glycerol is of great interest to the clinician and the laboratorian because TPN can contain large amounts of free glycerol: typically a TPN solution includes 3% glycerol (3000 mg/dL) (3). Because the patient in the present case had been on TPN, the possibility of a positive interference of glycerol in the TG measurement must be considered (4).
Shortly after admission to the first author's hospital, TPN was discontinued. Two days later the TG level had declined to 225 mg/dL. The marked and rapid decline in the TG level (1354 mg/dL to 225 mg/dL) in just 2 days raises the possibility that there was a positive interference in the original TG measurement from glycerol in the patient's TPN solution. To prove this hypothesis, a glycerol-blanked TG measurement would be required; however, this was not performed in the present case.
Certainly TG levels will decline with fasting and/or insulin treatment (which was not used in this case) (5); however, a greater than 80% decline in TG levels strictly due to fasting in 48 hours is unexpected favoring the possibility of a glycerol-induced positive interference in the initial TG measurement of 1354 mg/dL.
As with many situations, the pre-analytical state of the patient can have a huge effect on the measurements made in the clinical laboratory. Because most clinical labs do not use glycerol-blanked TG methods, the influence of a glycerol-induced positive interference in the TG measurement should be always be kept in mind while interpreting TG results especially in the setting of TPN treatment or diabetic ketoacidosis where glycerol levels can be elevated (6). There are also uncommon inborn errors of metabolism that can cause pseudohypertriglyceridemia such as glycerol kinase deficiency (7).
References
- Fortson MR , Freedman SN , Webster PD III . Clinical assessment of hyperlipidemic pancreatitis . Am J Gastroenterol. 1995; 90(12):2134-9.
- Bistrian BR. Update on total parenteral nutrition. Am J Clin Nutr 2001; 74:153-4.
- Yeo CJ, Cameron JL. The pancreas. In: Sabiston DC, Lyerly HK. eds. Textbook of surgery. The biological basis of modern surgical practice. Philadelphia: WB Saunders, 1997: 1151-86.
- Cole TG. Glycerol blanking in triglyceride assays: Is it necessary. Clin Chem 1990;36:1267-68.
- Twilla JD, Mancell J. Hypertriglyceridemia-induced acute pancreatitis treated with insulin and heparin. Am J Health Syst Pharm. 2012 Feb 1;69(3):213-6.
- Husband DJ, Pernet A, Gill GV, Hanning I, Alberti KG. The metabolic response to insulin deprivation in idiopathic brittle diabetes. Diabetes Res. 1986 May;3(4):193-8.
- Goussault Y, Turpin E, Neel D, Dreux C, Chanu B, Bakir R, Rouffy J. 'Pseudohypertriglyceridemia' caused by hyperglycerolemia due to congenital enzyme deficiency. Clin Chim Acta. 1982 Aug 18;123(3):269-74.