Mass spectrometry has had widespread adoption into the clinical laboratory due to its do-it-yourself adaptability and functionality as ‘gold standard’ testing [1, 2]. The current established diagnostic applications include toxicology, endocrinology, and microbial identification. Major advances have been made in mass analyzers, including instruments which combine upfront tandem mass spectrometry (MS/MS) with backend high-resolution time-of-flight (TOF) or Orbitrap technologies to provide accurate mass measurements below 1x10-4 daltons. This has allowed for the unique ability to perform untargeted data acquisition for unknown analytes from complex biospecimens such as plasma/serum and urine [3].
The applications of quadrupole high-resolution mass spectrometry (QHRMS) have been evolving at a dizzying pace and the clinical laboratory is just starting to adopt them into their assay repertoire. This Scientific Short aims to provide a simplified overview of QHRMS methodology, realistic current and emerging clinical applications, and limitations of bringing these techniques into the clinical arena.
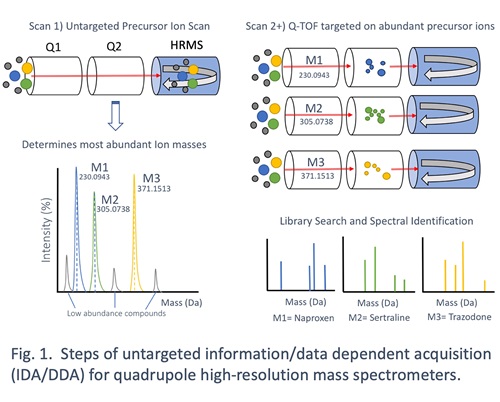
The main feature of QHRMS instruments is their capability to perform untargeted MS/MS data acquisition. The most common form of untargeted data acquisition is known as Information-Dependent Acquisition (IDA) or Data-Dependent Acquisition (DDA), depending on the instrument vendor. This form of untargeted data acquisition allows for the mass analyzer to parse unknown precursor ions for collision-induced dissociation and collect product ion spectra without a priori knowledge of what is being analyzed. Figure 1 demonstrates the series of steps involved in IDA/DDA – all rapidly taking place over relatively short cycle times (~1 second). In the first scan step, the MS/MS is bypassed to perform an accurate mass scan of precursor ions by the HRMS backend. From there, the instrument identifies the relative number and abundance of all analytes coming through in that cycle. The instrument then selects the top 10-20 most abundant precursor ions for successive MS/MS analysis and collects high-resolution product ion spectra. This cycle is repeated over and over during the chromatographic run. In the end, a data file containing all precursor ion scans and dependent product ion scans is generated for targeted data analysis from a curated spectral library of analytes, be they drugs, small molecule hormones, or physiologic metabolites.
Currently, clinical toxicology is the most prominent use of untargeted QHRMS for clinical testing. The utility comes from the fact that the desired analytes can be comprised of hundreds of exogenous compounds that require mostly qualitative detection. Large MS/MS spectral libraries for drugs, illicit substances, and natural products can be amassed, which are helpful as modified drugs and supplement trends appear over time. The untargeted manner of data acquisition allows for reanalysis in idiopathic cases with high suspicion of toxidrome without having to rerun the specimen. It can also be very useful for retrospective research for epidemiological aims.
Another promising utilization of QHRMS lies in testing for inborn errors of metabolism (IEM). These rare inherited deficiencies of various metabolic genes can lead to hundreds of classified diseases with a wide variety of clinical phenotypes. A subset of known IEMs are commonly screened for at birth, but rely on limited small molecule analyte panels for identification. Untargeted metabolomics has the potential to survey a much larger range of metabolic perturbations for both improving diagnosis and novel biomarker discovery. In an ongoing partnership between Baylor College of Medicine and Metabolon, untargeted metabolomic IEM testing has demonstrated both clinical utility and a model for validating an MS-based ‘omics’ to clinical standards [4-6].
Lastly, endocrine testing for steroid hormones has had a significant push to employ mass spectrometry for both diagnosis and basic science [7, 8]. Large numbers of small molecule hormones can be dysregulated in endocrine disorders, yet testing is generally performed in sequential algorithmic workup. One group out of the Mayo Clinic has demonstrated utility for a clinically validated QHRMS method profiling 26 steroid hormones in urine for the diagnosis of adrenal disorders [9]. It should be noted, this method is able to survey, distinguish, and quantify highly similar analytes based on the high-resolution of the precursor ion scan without utilizing DDA/IDA. This ability to perform multiplex and quantitative endocrine profiling from a single non-invasive sample has the potential to greatly improve time to diagnosis and simplify testing algorithms in clinical settings.
The limitations for exploring QHRMS methods in the clinical setting boil down to the actual need for such broad testing capabilities in a laboratory’s patient population and the budget, staffing, and technical skill to perform ongoing testing. To address the first point, the majority of clinical labs do not perform experimental toxicology or endocrine testing; this is largely reserved for tertiary care centers with research-oriented clinicians and laboratorians. For our laboratory in particular, QHRMS toxicology testing has been highly utilized in collaboration with our clinical toxicologists who dually staff the poison control center for Northern California’s catchment area. In other scenarios, including community hospitals or high-throughput reference laboratories, such esoteric testing may neither be needed nor highly utilized. The second consideration should be the high cost and effort investments in establishing and maintaining a method. The upfront costs of a QHRMS instruments are in the hundreds of thousands of US dollars with annual service contracts in the tens of thousands. Additionally, few clinical laboratories in the US perform QHRMS testing making training opportunities scarce for clinical laboratory staff and directors alike. All this being said, if there is a nidus to bring this testing in-house, the adaptability and scalability of this tool is immense. If more clinical laboratories choose to bring on QHRMS testing there will be greater exposure to the clinical community and a greater diversity of applications to explore.
In summary, QHRMS represents the forefront of mass spectrometry technologies in the clinical laboratory due to their ability to perform untargeted MS/MS data acquisition for broad analysis of any number of small molecule analytes. The applications of this testing are mainly restricted to toxicology, but future widespread adoption may emerge for IEM and endocrine testing. These methods are highly adaptable for rapidly developing methods compared to traditional tandem mass spectrometry, but may be too specialized for the majority of clinical needs. QHRMS has only started to make an appearance into the clinical laboratory over the past decade, so the scope and scale of this cutting-edge technology in diagnostic assays will be determined with time.
References
- Jannetto PJ, Fitzgerald RL. Effective Use of Mass Spectrometry in the Clinical Laboratory. Clin Chem 2016; 62(1): 92-8.
- Clarke W. Chapter 1 - Mass spectrometry in the clinical laboratory: determining the need and avoiding pitfalls. In: Nair H, Clarke W. Mass Spectrometry for the Clinical Laboratory. San Diego: Academic Press, 2017:1-15.
- Wu AH, Gerona R, Armenian P, French D, Petrie M, Lynch KL. Role of liquid chromatography-high-resolution mass spectrometry (LC-HR/MS) in clinical toxicology. Clin Toxicol (Phila) 2012; 50(8): 733-42.
- Miller MJ, Kennedy AD, Eckhart AD, et al. Untargeted metabolomic analysis for the clinical screening of inborn errors of metabolism. J Inherit Metab Dis 2015; 38(6): 1029-39.
- Kennedy AD, Wittmann BM, Evans AM, et al. Metabolomics in the clinic: A review of the shared and unique features of untargeted metabolomics for clinical research and clinical testing. J Mass Spectrom 2018; 53(11):1143-54.
- Burrage LC, Thistlethwaite L, Stroup BM, et al. Untargeted metabolomic profiling reveals multiple pathway perturbations and new clinical biomarkers in urea cycle disorders. Genet Med 2019; 21(9): 1977-86.
- Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab 2013; 98(10): 3971-3.
- Auchus RJ. Steroid assays and endocrinology: best practices for basic scientists. Endocrinology 2014; 155(6): 2049-51.
- Hines JM, Bancos I, Bancos C, et al. High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin Chem 2017; 63(12): 1824-35.