Multianalyte assays with algorithmic analyses (MAAAs) are tests that incorporate results from a panel of tests (molecular or non-molecular), with or without other clinical information, into an algorithm in order to generate a risk or probability score.
Clinical adoption of tests with algorithmic analyses dates back to the 1980s, with the maternal serum screening for risk assessment for conditions such as Down Syndrome among the pioneer tests of this growing field (1). The number of MAAAs have boomed, but where are we at with their clinical adoption across clinical applications?
MAAAs are often unique to a private laboratory or manufacturer, mostly because of the proprietary nature of the algorithmic component, and commonly offered as laboratory developed tests (LDTs). Yet, few MAAAs are US Food and Drug Administration (FDA) cleared. The availability of tests, such as the Ovarian Malignancy Algorithm (ROMA)TM, Prostate Health Index (phi) and Nephrocheck® in analysis routinely used in clinical laboratories, increases the feasibility of widespread use.
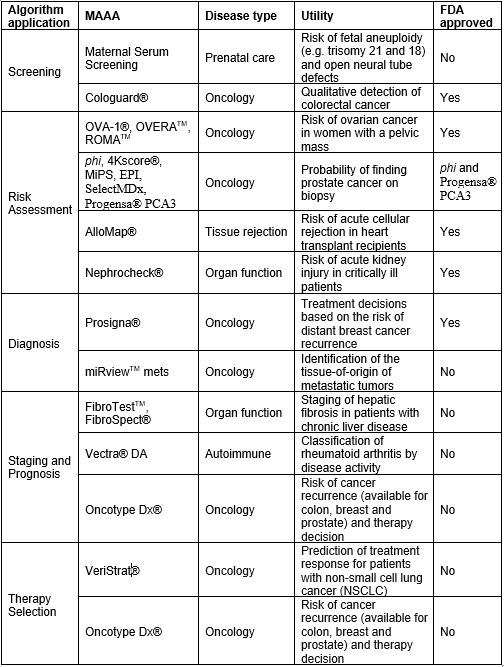
Generally speaking, algorithmic tests show diagnostic superiority in diseases in which single biomarkers have limited validity or a non-invasive biomarker is lacking. For example, measuring PSA for prostate cancer screening is standard of care but its validity has been extensively scrutinized because of the high number of false positives leading to unnecessary biopsies. A wide collection of MAAAs (e.g. phi, 4KScore® and Mi-Prostate Score (MiPS)) claim to improve screening of prostate cancer by predicting the risk of high-grade cancer in men with modest elevations of PSA, and by guiding the decision to proceed to biopsy (2). Likewise, tests such as OVA-1®, OVERATM, and ROMATM help predict the risk of having ovarian cancer in women with a pelvic mass (3), information subsequently used to manage care decisions. Similarly, other MAAAs are clinically established across diagnostic applications and disease states (Table 1). New algorithmic scores are also emerging for conditions like preeclampsia, acute coronary syndrome and sepsis.
Last year, we discussed MAAAs during the American Association for Clinical Chemistry (AACC) 70th Annual Scientific Meeting (4). In preparation for our session, we posted a survey in the AACC Artery (5) to ask AACC members about their laboratory experience with MAAAs. Most of the 23 respondents represented hospital and academic laboratories and 57% did not offer any MAAA. Labs who offered them, listed prenatal screening most commonly, followed by ROMA, phi and a miscellaneous MAAAs; only half offered them in-house. Merely 5 respondents planned on expanding their MAAA menu in the next 3-5 years. Despite limitations of low participation and the informal nature of the survey, the responses of the laboratory community pointed to slow clinical adoption of MAAAs.
It is prudent to evaluate the clinical evidence of established and emerging MAAAs prior to implementation and to drive awareness of potential analytical limitations (e.g. entering test results in online calculators) and optimal test interpretation (e.g. cut-off used). Although there is general consensus on the value of using an MAAA to generate a diagnostic risk score for certain clinical applications, there is presently no published consensus on their use and wide clinical adoption is still ongoing (1). This underscores the need of additional clinical validation, evidence-based and utilization guidance, informatics resources, and reimbursement so these can become standard of care where appropriate.
Table 1: Representative MAAAs across diagnostic applications and disease types
References:
- Colón-Franco JM, et al, Current and Emerging Multianalyte Assays with Algorithmic Analyses—Are Laboratories Ready for Clinical Adoption?, Clinical Chemistry, 2018; 64: 64-6
- Kohaar I, Petrovics G, and Srivastava S, A Rich Array of Prostate Cancer Molecular Biomarkers: Opportunities and Challenges, Int. J. Mol. Sci. 2019; 20, 1813
- Turner KA and Algeciras-Schimnich A, Multianalyte Assays With Algorithmic Analysis in Women’s Health, Clinical Laboratory News, July 2018
- https://www.myadlm.org/meetings-and-events/resources-from-past-meetings, accessed June 15, 2019
- https://artery.aacc.org, accessed May 31, 2019