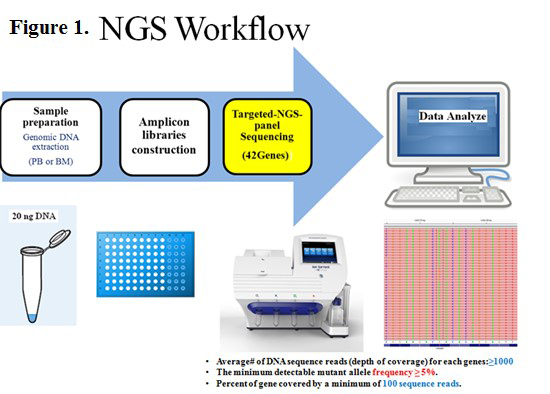
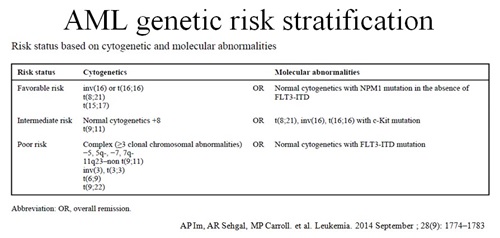
Somatic mutations have been implicated in the pathogenesis of acute myeloid leukemia (AML). In the past, risk stratification was accomplished by cytogenetic analysis, but with the development of molecular technology, the classification and prognosis of AML increasingly depend on both cytogenetic and molecular abnormalities. Mutation screening in AML is an integral part of risk stratification to guide therapy and monitor disease response/relapse. Next Generation Sequencing (NGS) can simultaneously detect multiple mutations in leukemia.
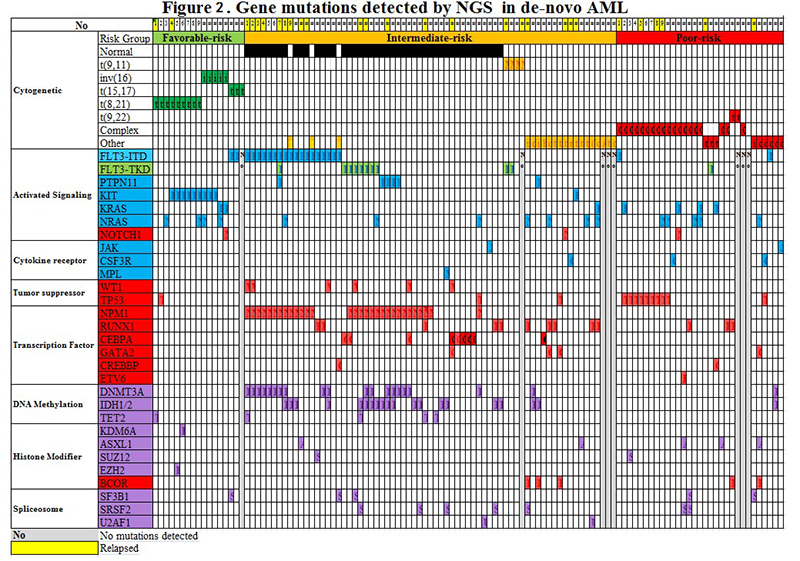
We report mutations in AML using targeted NGS. The study included 117 de novo AML patients from the pathology department/Knight diagnostic laboratory/Oregon Health and Science University (OHSU) (2013-2015). The targeted-NGS-panel covered 42 genes relevant to hematopoietic malignancies. As D shown in Figure 2, 93.2% (109/117) of AML cases showed at least one mutation detected by NGS. In the intermediate-risk-cytogenetic-group, the most common mutations include transcriptional factors at the rate of 71% (49/69). Mutations in epigenetic-regulators and splicing-genes were less frequent (56.5%, 39/69). Multiple mutations (>2) were often seen (66.7%, 46/69). The FLT3-ITD (66.7%, 12/18) and NPM1 (80%, 24/30) mutations occurred with epigenetic-regulators (DNMT3A, IDH and TET2) at a high frequency. In the favorable-risk-cytogenetic-group, KIT (52.9%, 9/17) mutations were frequent. In the poor-risk-cytogenetic-group, P53 (32.3%, 10/31) and RAS (25.8%, 8/31) mutations were common. Mutations with both DNMT3A and FLT3-ITD showed the high relapse rate (60%, 6/10).
AML is a heterogeneous disease with variable treatment outcome. Numerous recurrent mutations have been found and are used for risk stratification, targeted therapy and monitoring therapeutic response. This study demonstrates that targeted NGS mutation analysis is applicable in the clinical setting and provides a better understanding of leukemogenesis.