The oral anticoagulant warfarin (also called coumadin) is a vitamin K antagonist that is widely used to prevent venous thrombosis. Warfarin inhibits the post-translational carboxylation of coagulation factors II, VII, IX and X. This diminishes the ability of these factors to interact with phospholipid membranes. The degree of anticoagulation achieved by warfarin is monitored by the common coagulation test known as the prothrombin time (PT). The latter is a clot-based test carried out on citrated plasma. The PT is initiated by adding tissue factor in (supraphysiologic concentrations) along with phospholipids and calcium chloride. The tissue factor/phospholipid combination is called thromboplastin. The PT does not prolong in response to deficiencies all the coagulation factors - for example, it is unaffected by low FVIII & FIX in patients with hemophilia.
The international normalized ratio (INR) was introduced in an attempt to standardize the PT. In its original manifestation, the PT was very variable because different thromboplastins were non-standardized and derived from many varied sources. PTs performed on the identical specimen by different laboratories were inconsistent. The concept behind the INR is that differences between the thromboplastins are accounted for by a calculation:-
INR = [PTpatient ÷ MNPT]ISI
The INR has no units (it is a ratio) and is determined to one decimal place. The first step of the INR calculation is to “normalize” the PT by comparing it to the mean normal prothrombin time (MNPT), the geometric mean of the prothrombin times of the healthy adult population. In the second step, this ratio is raised to a power designated the ISI, or international sensitivity index. The ISI is a function of the thromboplastin reagent. Two groups of data are used to derive the ISI (i) normal healthy individuals and (ii) patients stabilized on warfarin. Paired PT data are obtained from multiple samples using both the working thromboplastin reagent and the international thromboplastin standard.
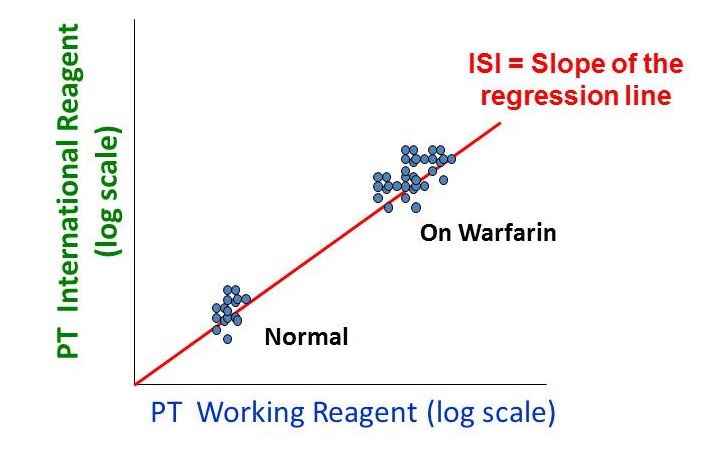
The normal INR is typically 0.9 to about 1.1. On warfarin therapy, the INR elevates to between 2 and 3.5 and most hospital pharmacies and clinical hematology services will have specific INR goals documented in their treatment protocols.
The determination of the ISI does not include patients where the PT is prolonged due to, for example, severe liver disease. Indeed, the relationship between normal vs. warfarin-treated patients shown in the figure above, makes no prediction at all about the relationship between the working thromboplastin reagent and the WHO standard in liver disease. Put simply, if there is a certain degree of anticoagulation and a certain risk of bleeding present at an INR of 3.8 in a patient on warfarin, it does not necessarily follow that the same holds true for a patient with liver failure who has an INR of 3.8. Indeed, the elevated INR in liver disease does not protect patients against deep venous thrombosis.
This may be more apparent if one reexamines coagulation physiology. In warfarin therapy, concentrations of vitamin K-dependent clotting factors are decreased, as determined by activity assays. The anticoagulant factors protein C and S are also decreased. While all this may also be true for liver disease, other factors will be affected, including low factors V, XI, low fibrinogen and low antithrombin III. Typically, FVIII and von Willebrand factor are elevated in liver disease.
In summary:
- The prothrombin time is a surrogate for the effects of warfarin therapy
- A normal PT does not exclude a bleeding tendency (e.g., hemophilia)
- The INR is a standardized prothrombin time designed to account for differences in thromboplastin.
- Derivation of the ISI is based on patients receiving warfarin
- The INR does not predict bleeding tendency in patients with liver failure or other systemic diseases.