Summary
DOI: 10.1373/clinchem.2016.265124
A 69-year-old man was referred to the endocrine clinic with a 3-year history of erectile dysfunction, reduced libido, and lack of nocturnal tumescence with no response to phosphodiesterase type 5 inhibitors (sildenafil and tadalafil). The symptoms troubled him to such an extent that he asked his general practitioner to be referred to a specialist clinic.
Student Discussion
Student Discussion Document (pdf)
Ingrid Borovickova,1* Naomi Adelson,1 Ananth Viswanathand,2 and Rousseau Gama1
Departments of 1Biochemistry and 2Endocrinology, New Cross Hospital, The Royal Wolverhampton NHS Trust, Wolverhampton, UK.
*Address correspondence to this author at: Biochemistry Department, New Cross Hospital, The Royal Wolverhampton NHS Trust, Tallaght Dublin 24, Ireland. Fax +353-01-4143931; e-mail: [email protected]
Case Description
A 69-year-old man was referred to the endocrine clinic with a 3-year history of erectile dysfunction, reduced libido, and lack of nocturnal tumescence with no response to phosphodiesterase type 5 inhibitors (sildenafil and tadalafil). The symptoms troubled him to such an extent that he asked his general practitioner to be referred to a specialist clinic.
The patient had been through a normal puberty. Although he fathered no children, he was unconcerned about this and never sought fertility investigation or treatment. His past medical history was clinically significant for newly diagnosed interstitial lung disease owing to hypersensitivity pneumonitis, osteoarthritis, and gastroesophageal reflux; his only medications were ibuprofen gel and lansoprazole. He was never prescribed steroids, ketoconazole, or spironolactone. He had not undergone ionizing radiation and denied using over-the-counter or recreational drugs. He was an ex-smoker who drank 8 units of alcohol weekly. He did not recall a prior history of mumps or testicular trauma. He was unaware of any family members who had an autoimmune disorder or fertility issues.
On examination he was 178 cm tall and obese [body mass index (BMI) 37.3 kg/m2]. His arm-span-to-height ratio was <1.05 and his cardiovascular examination did not reveal heart murmurs. He had a normal hair pattern and no gynecomastia. Testicular volume was reduced bilaterally at 12–15 mL (reference interval ≥15 mL).
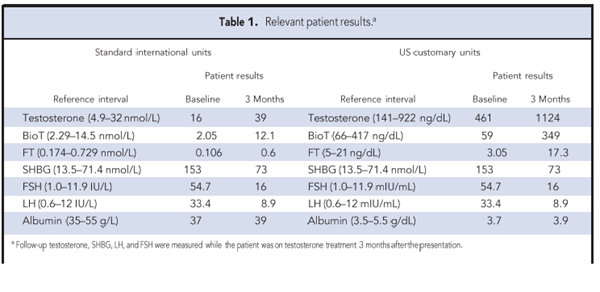
Testosterone, measured by a 1-step chemiluminescent immunoassay (Abbott Architect, second generation testosterone assay) was 16.0 nmol/L (reference interval 4.9–32 nmol/L); his sex hormone–binding globulin (SHBG)3 was increased at 153 nmol/L (13.5–71.4 nmol/L) as were luteinizing hormone (LH) and follicle-stimulating hormone (FSH) at 33.4 IU/L (0.6 –12.0
IU/L) and 54.7 IU/L (1.0 –11.9 IU/L), respectively (Table 1). These results, which indicated hypergonadotropic hypogonadism, were confirmed on repeated testing 3 weeks later. At that time, testosterone was also measured by LC-MS/MS and the results confirmed a normal total testosterone, thus excluding a positive immunoassay interference. Low calculated values of bioavailable testosterone (bioT) [2.05 nmol/L (2.29 –14.5 nmol/L)] and free testosterone (FT) [0.106 nmol/L (0.174–0.729 nmol/L)] were consistent with hypogonadism. Further blood tests showed normal results for the routine metabolic panel, complete blood count, transferrin saturation, estrogen, thyroid tests, and prolactin. Plasma glucose, adjusted calcium, vitamin B12, and morning cortisol results were also unremarkable, making an autoimmune condition unlikely.
After discussion with the patient, transdermal testosterone replacement was initiated. At followup
3 months later, his total testosterone had increased to 39 nmol/L; this was mirrored by a
reduction in SHBG (73 nmol/L) and gonadotropins (FSH 16.0 IU/L, LH 8.9 IU/L). His
libido, general well-being, and erectile function improved substantially. The etiology of his
hypergonadotropic hypogonadism could not be further elucidated because of unexpectedly rapid
progression of his interstitial lung disease leading to oxygen dependency. He declined further
investigations and eventually stopped taking testosterone replacement upon learning of a poor
prognosis of his rapidly progressing lung disease.
Questions to Consider
- What are the criteria for LOH?
- What are the pitfalls of measuring serum total testosterone
concentration?
- What common conditions cause increased SHBG?
- What methods should be used to determine FT and
bioT?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the August 2017 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2016.265124
Copyright © 2017 American Association for Clinical Chemistry