Summary
DOI: 10.1373/clinchem.2016.264507
A 27-year-old man was found unconscious in his car by police. He was taken to an outside hospital where results of his serum alcohol and initial urine drug screen were reportedly negative. On arrival to our hospital, the patient was awake, partially oriented, and able to follow simple commands.
Student Discussion
Student Discussion Document (pdf)
Brian N. Chang1 and Michael P. Smith1,2*
1Beaumont Health System, Royal Oak, MI; 2Oakland University William Beaumont School of Medicine, Rochester Hills, MI.
*Address correspondence to this author at: Beaumont Hospital–Royal Oak, 3601 W. 13 Mile Rd., Royal Oak, MI 48073. Fax 248-551-0557; e-mail [email protected].
Case Description
27-year-old man was found unconscious in his car by police. He was taken to an outside hospital where results of his serum alcohol and initial urine drug screen were reportedly negative. On arrival to our hospital, the patient was awake, partially oriented, and able to follow simple commands. He reported feeling sedated and “loopy,” was unable to remember any events before hospitalization, and was an overall poor historian. He denied illicit drug use, suicidal ideation, homicidal ideation, or any physical complaints. He did express delusions including that he “was an alien with green blood.” Past medical history was significant for schizophrenia with continued auditory hallucinations secondary to noncompliance with risperidone therapy. A review of the medical record revealed a prescription for tramadol.
Vital signs were normal save for borderline tachycardia and the physical exam was noncontributory. The patient’s complete metabolic panel was significant for mild hypokalemia (potassium 3.1 mmol/L, reference interval 3.5–5.2 mmol/L), hypophosphatemia (phosphorous 0.7 mg/dL, reference interval 2.3– 4.3 mg/dL), slightly increased aspartate aminotransferase (60 U/L, reference interval 10–37 U/L), and indirect bilirubinemia (3.7 mg/dL, reference interval 0.3–1.2 mg/dL). The initial troponin I concentration was 0.16 ng/mL (reference <0.06 ng/mL) that peaked at 0.21 ng/mL (≥0.20 ng/mL suggestive of myocardial damage) approximately 5 hours later. His creatine kinase (CK)3 was 1017 U/L (reference interval 40–230 U/L). Serial electrocardiograms were performed and showed evidence of left axis deviation but were otherwise unremarkable.
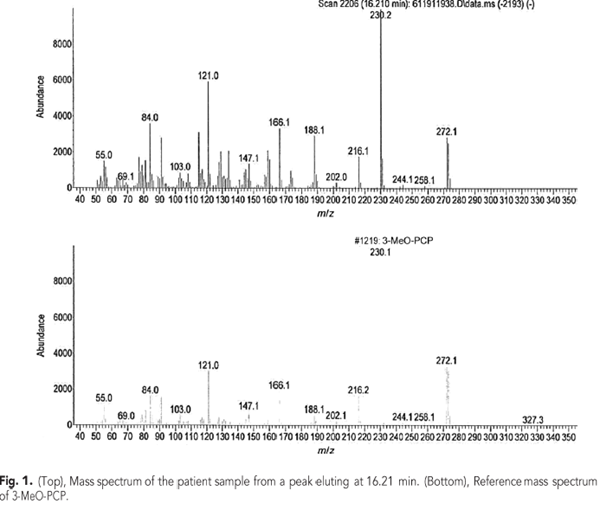
The patient was admitted for observation and treated with intravenous fluids. A transthoracic echocardiogram was performed and did not show any evidence of wall motion abnormality. On the second day of hospitalization, he was deemed medically stable with a plan to resume risperidone therapy as an outpatient. His CK had trended downward to 403 U/L by the time of discharge. A urine drug screen was performed upon admission using the Abbott Architect c4000 analyzer (Abbott Diagnostics). The test menu consisted of amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, methadone, opiates, and phencyclidine (PCP) screens (Abbott Diagnostics). The only positive result was for PCP (cutoff of 50 ng/mL). Confirmation testing was performed using an Agilent 7890A/5975C GC-MS (Agilent Technologies) in full scan mode. No PCP was detected; however, the presence of 3-methoxyphencyclidine (3-MeO-PCP) (Fig. 1) was confirmed. No other drugs, including tramadol, were detected.
Questions to Consider
- Very few drugs of abuse have a specific antidote (e.g., naloxone, N-acetyl-cysteine);
however, why is it still clinically relevant to undertake emergent testing for the
many that do not?
- Excluding administrative and technical error, how might one explain a negative
GC-MS confirmatory result performed at an outside laboratory on a screen-positive
sample that is nonetheless highly likely to be a true positive?
- Given the proliferation of NPSs where immunoassay kits are unavailable could high
throughput mass spectrometry platforms fill the void?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the May 2017 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2016.264507
Copyright © 2017 American Association for Clinical Chemistry