Summary
DOI: 10.1373/clinchem.2014.225847
A 78-year-old man with prostate cancer needed intravenous treatment with amikacin for a suspected gram-negative bacillary respiratory infection. He had a body surface area of 1.97 m2, weight of 78 kg, and body mass index of 29 kg/m2.
Student Discussion
Student Discussion Document (pdf)
Noemí Rebollo1* and Francisco Javier Cepeda-Piorno2
1Pharmacy Service and 2Clinical Laboratory, Santos Reyes Hospital, Aranda de Duero, Spain.
*Address correspondence to this author at: Santos Reyes Hospital, 6 Ruperta Baraya Ave., Aranda de Duero, Spain 09400. Fax +34-947-522018; E-mail [email protected].
Case Description
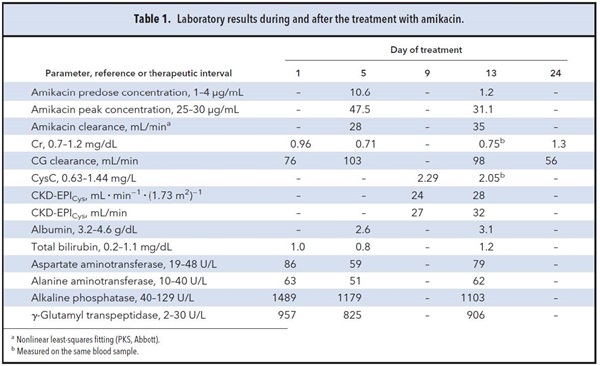
A 78-year-old man with prostate cancer needed intravenous treatment with amikacin for a suspected gramnegative bacillary respiratory infection. He had a body surface area of 1.97 m2, weight of 78 kg, and body mass index of 29 kg/m2. Laboratory test results are shown in Table 1. Because the initial renal function was judged to be normal and the patient was considered overweight, the antibiotic was empirically administered at 12 mg/kg once daily (1000 mg/24 h).
Predose and peak concentrations (obtained 0.5 h after completion of the intravenous dose of amikacin) were measured by a homogeneous immunoassay (Cobas Integra®, Roche) to confirm that the dosage was correct. Concentrations were fitted to a 1-compartment model using Bayesian analysis (PKS®, Abbott). Because the new estimated glomerular filtration (GFR) rate equations, such as Modification of Diet in Renal Disease–isotope dilution mass spectrometry (MDRD-IDMS) or CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), are not included in the software, estimations for dose adjustment were carried out using the creatinine clearance (CrCl) calculated by the Cockcroft-Gault (CG) formula. Serum creatinine (sCr) was measured by the compensated Jaffe kinetic method (IDMS-traceable assay). Adjustment of aminoglycoside dosage sought to maintain concentrations within the therapeutic intervals.
Fig. 1 shows amikacin concentrations determined in our patient as well as the dosage regimens. On the fifth day from the beginning of the antibiotic treatment, the amikacin volume of distribution and clearance estimated by applying the population pharmacokinetic equations and taking into account the CrCl were 0.25 (0.07) L/kg and 0.04 (0.01) mL · min-1 · kg-1, respectively. The predose concentration predicted from these parameters was 0.3 μg/mL, which was lower than the observed concentration in our patient (10.6 μg/mL). The presence of drug interactions with comedications was ruled out by using the Lexi-Interact™ online database. However, the patient also suffered from liver injury (Table 1). Because it has been proven that in advanced liver diseases or liver cirrhosis an overestimation of the GFR occurs when sCr is used, the measurement of serum cystatin C (CysC) was recommended to detect a possibly impaired renal function. Pending this measurement, a new dosing scheme of 750 mg/36 h was recommended.
Case Follow Up
Serum CysC was 2.29 mg/L, measured by an immunonephelometric method (Immage®, Izasa) on the ninth day. The GFR calculated by using the CKD-EPICys formula was 24 mL · min-1 ·
(1.73 m2)-1, corresponding to 27 mL/min according to the body surface area of the patient. This value was very similar to the estimated amikacin clearance on the fifth day (28 mL/min).
Another pharmacokinetic control was carried out on the 13th day and the observed predose and peak concentrations were considered to be adequate. CysC was also measured the same day and a CKD-EPICys of 28 mL · min-1 · (1.73 m2)-1 was estimated. To evaluate the performance of the Bayesian estimation, the measured amikacin concentrations were compared with the predicted ones, generated on the basis of the estimates of the CrCl and that estimated by the CKD-EPICys equation. The predicted (SD) concentrations were 1.11 (0.6) and 30.40 (3.30) μg/mL by using the sCr-based estimates, and 1.25 (0.71) and 34.02 (3.95) μg/mL by using CysC-based estimates. Prediction of amikacin serum concentration was more accurate when the estimation was carried out by entering the CKD-EPICys (r2, 3.2835 vs 1.6009).
An increase of Cr (1.3 g/dL) was observed 9 days after the end of the treatment with amikacin, which was compatible with the presence of acute kidney injury.
Questions to Consider
- Under what circumstances is Cr a poor predictor of renal function and leads to overestimation of GFR?
- If renal impairment is suspected and Cr is not increased, what other marker is suggested by Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines to estimate GFR?
- What family of antibiotics could also be considered as markers of renal function?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the June 2015 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2014.225847
Copyright © 2015 American Association for Clinical Chemistry