Summary
DOI: 10.1373/clinchem.2014.227819
A 62-year-old woman noticed muscle weakness in the right leg, which was followed a few months later by weakness in the left leg. Over a 6-month period, the muscle weakness progressed to the upper limbs. One year after onset, the patient was referred to a neurologist with a suspected diagnosis of amyotrophic lateral sclerosis.
Student Discussion
Student Discussion Document (pdf)
Laurens Dobbels,1 Philip Van Damme,1 Michael Mahler,2 and Xavier Bossuyt3,4*
Departments of 1Neurology and 3Laboratory Medicine, University Hospitals Leuven, Leuven, Belgium; 2Inova Diagnostics, Inc., San Diego, CA; 4Department of Microbiology and Immunology, Katholieke Universiteit Leuven, Leuven, Belgium.
*Address correspondence to this author at: Laboratory Medicine, University Hospitals Gasthuisberg, Herestraat 49, 3000 Leuven, Belgium. Fax +32-16-34-79-31; E-mail: [email protected].
Case Description
A 62-year-old woman noticed muscle weakness in the right leg, which was followed a few months later by weakness in the left leg. Over a 6-month period, the muscle weakness progressed to the upper limbs. One year after onset, the patient was referred to a neurologist with a suspected diagnosis of amyotrophic lateral sclerosis. She was taking atorvastatin 40 mg (started approximately 6 years earlier) and L-thyroxine 125 μg (for hypothyroidism after nuclide therapy for Graves disease). Clinical examination revealed a proximal paresis in the arms and legs. Creatine kinase (CK) activity was 7925 U/L (reference interval <145 U/L). Electromyography showed myogenic changes (small and polyphasic motor units). A toxic statin myopathy was considered, although the time span between the start of the statin and onset of the symptoms was long. Late-onset Pompe (glycogen storage) disease was excluded by α-glucosidase activity testing. Further laboratory testing was unremarkable. A biopsy of the right quadriceps muscle was performed and revealed polygonal muscle fibers of varying diameter (and an increase of internal nuclei). There was some evidence of endomysial fibrosis without inflammatory infiltration.
Individual muscle fibers showed necrosis with myocyte phagocytosis and without HLA-ABC upregulation.
Patient Follow-up
The muscle weakness was progressive and also affected distal hand muscles and bulbar muscles. The use of statins was discontinued, and high-dose intravenous methylprednisolone was given, followed by oral tapering. Ten days later, there was only minor improvement of the muscle weakness, and azathioprine 100 mg per day was added. Ezetimibe and fenofibrate were started for hypercholesterolemia. The weakness improved, CK activity decreased, and oral methylprednisolone was stopped. The patient fully recovered and was stable at 1-year follow-up after discontinuation of azathioprine. The CK activity normalized.
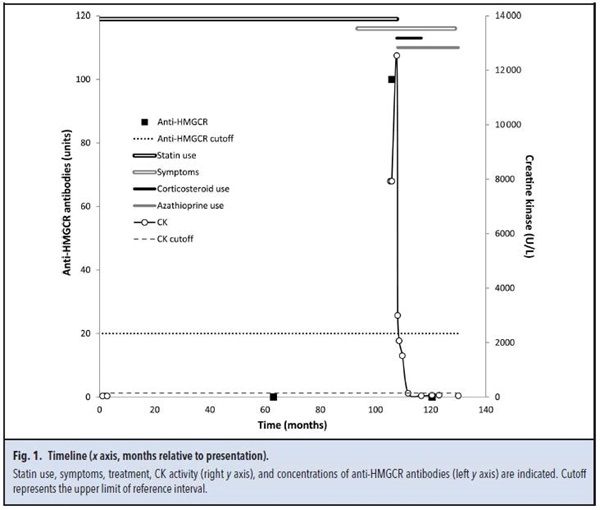
Quantification of antibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) was not available at the time the patient presented but was performed later (with approval of the local ethics committee) by use of ELISA (Quanta Lite HMGCR, Inova Diagnostics) on 3 banked serum samples obtained (a) before onset of symptoms, (b) when the patient presented with muscle weakness, and (c) after treatment. Anti-HMGCR antibodies were absent before symptoms and increased to 100 U (cutoff 20 U) when the patient presented with symptoms. The antibodies decreased to 0.8 U after immunosuppressive treatment. Fig. 1 shows a timeline of symptoms, treatment, CK, and anti-HMGCR antibodies.
Questions to Consider
- What are potential etiologies of necrotizing myopathy?
- What laboratory tests can be used to help determine the etiology?
- What drugs are associated with myopathy?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the September 2015 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2014.227819
Copyright © 2015 American Association for Clinical Chemistry