Summary
DOI: 10.1373/clinchem.2011.168393
A 90-year-old man was admitted to the Emergency Department with severe congestive heart failure and kidney failure. His medical history included successful surgery for prostatic carcinoma, aortocoronary bypass surgery, and cardiac pacemaker implantation. The patient was regularly taking digoxin, enalapril, aspirin, transdermal nitrate, and furosemide.
Student Discussion
Student Discussion Document (pdf)
Dominika Szoke,1,2* Alberto Dolci,1,2 Augusto Genderini,3 and Mauro Panteghini1,2
1Clinical Biochemistry Laboratory, “Luigi Sacco” University Hospital, Milan, Italy; 2Department of Clinical Sciences, University of Milan Medical School, Milan, Italy; 3Nephrology and Dialysis Department, “Luigi Sacco” University Hospital, Milan, Italy.
*Address correspondence to this author: Clinical Biochemistry Laboratory, “Luigi Sacco” University Hospital, Via GB Grassi 74, Milan 20157, Italy.
Fax 39-02-5031-9835; E-mail: [email protected].
Case Description
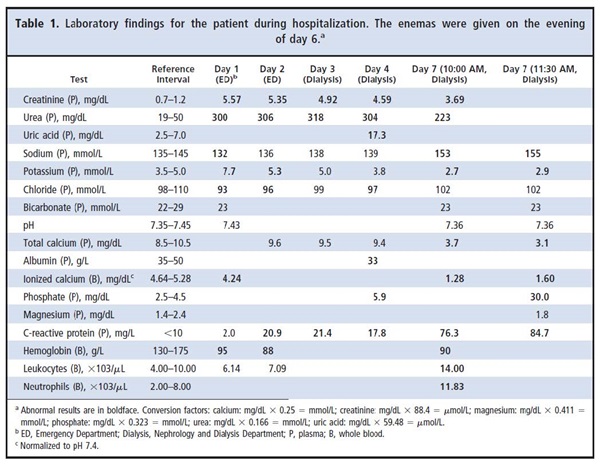
A 90-year-old man was admitted to the Emergency Department with severe congestive heart failure and kidney failure. His medical history included successful surgery for prostatic carcinoma, aortocoronary bypass surgery, and cardiac pacemaker implantation. The patient was regularly taking digoxin, enalapril, aspirin, transdermal nitrate, and furosemide. At admission, the patient was hyperkalemic (Table 1), and therapy with sodium polystyrene sulfonate and ethacrynic acid was started immediately. The diuretic therapy was continued after his admission to the Nephrology and Dialysis Department on the second day of hospitalization. An ultrasound examination in which the kidneys appeared small and hyperechoic confirmed chronic renal impairment. On the evening of the sixth day of hospitalization, 2 enemas (120 mL each) were administrated 30 min apart to relieve prolonged constipation. The patient vomited the following night, and a nasogastric tube was inserted. The next morning, the patient showed signs of dehydration and was hypotensive (blood pressure, 90/40 mmHg). The patient’s abdominal distension prompted an abdominal radiograph, which showed signs of intestinal obstruction. Laboratory findings revealed severe hypocalcemia [3.7 mg/dL (0.93 mmol/L); reference interval, 8.5–10.5 mg/dL (2.13–2.63 mmol/L)] and alterations in the plasma concentrations of other major plasma ions (Table 1). An intravenous infusion of calcium gluconate was started immediately, and an abdominal computed tomography evaluation was requested. The severe electrolyte abnormalities were confirmed after analysis of a second blood sample drawn after 1.5 h, which revealed severe hyperphosphatemia
[30.0 mg/dL (9.69 mmol/L); reference interval, 2.5–4.5 mg/dL (0.81–1.45 mmol/L)] without signs of overt acidosis. In the meantime, the abdominal computed tomography scan revealed paralytic ileus. Emergent hemodialysis was planned, but despite intensive
treatment, the patient’s electrocardiogram showed an increased QT interval. He finally went into cardiac arrest and died before hemodialysis could begin.
Questions to Consider
- Which laboratory tests are useful in the evaluation of a patient with severe hypocalcemia?
- What are several causes of severe hypophosphatemia?
- What treatment should be used in patients with severe hypophosphatemia and hypocalcemia?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the November 2012 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2011.168393
Copyright © 2012 American Association for Clinical Chemistry