Summary
DOI: 10.1373/clinchem.2010.148718
A 9-year-old girl was admitted to our hospital with juvenile metachromatic leukodystrophy (arylsulfatase A deficiency). Symptoms of this lysosomal storage disease, such as decreased school performance and compromised motor skills, had started 1 year earlier. She underwent bone marrow transplantation from a matched unrelated donor after receiving conditioning with fludarabine, treosulfan, and thiotepa, together with thymoglobulin, a rabbit antithymocyte globulin. Conditioning was well tolerated, and the posttransplantation period was uneventful except for 1 febrile episode. Graft-vs-host disease (GvHD)4 prophylaxis consisted of 3 methotrexate doses in combination with a starting daily cyclosporin A (CsA) dosage of 3 mg · kg−1 · day−1.
Student Discussion
Student Discussion Document (pdf)
Andreas Peter,1* Maria Shipkova,2 Eberhard Wieland,2 Erwin Schleicher,1 and Ingo Müller3
1Department of Internal Medicine, Division of Endocrinology, Metabolism, Pathobiochemistry and Clinical Chemistry, University of Tübingen, Tübingen, Germany; 2Central Institute for Clinical Chemistry and Laboratory Medicine, Klinikum Stuttgart, Stuttgart, Germany; 3Department of General Paediatrics, Haematology, Oncology, University Children’s Hospital, Tübingen, Germany.
*Address correspondence to this author at: Department of Internal Medicine, Division of Endocrinology, Metabolism, Pathobiochemistry and Clinical Chemistry, University of Tübingen, Otfried-Müller-Str. 10, 72076 Tübingen, Germany. Fax +49-7071-294582; e-mail [email protected].
Case Description
A 9-year-old girl was admitted to our hospital with juvenile metachromatic leukodystrophy (arylsulfatase A deficiency). Symptoms of this lysosomal storage disease,
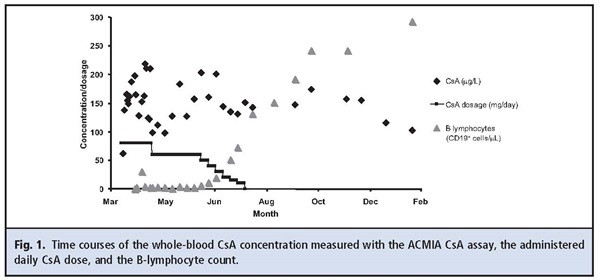
such as decreased school performance and compromised motor skills, had started 1 year earlier. She underwent bone marrow transplantation from a matched unrelated donor after receiving conditioning with fludarabine, treosulfan, and thiotepa, together with thymoglobulin, a rabbit antithymocyte globulin. Conditioning was well tolerated, and the posttransplantation period was uneventful except for 1 febrile episode. Graft-vs-host disease (GvHD) prophylaxis consisted of 3 methotrexate doses in combination with a starting daily cyclosporin A (CsA) dosage of 3 mg ·ıkg -1 · day -1. Trough CsA concentrations were monitored with the antibody-conjugated magnetic immunoassay (ACMIA) for CsA (RxL Dimension; Siemens). CsA concentrations entered the therapeutic interval after 3 days, and the dosage was adjusted to achieve trough concentrations of 120–150 μg/L (Fig. 1). The patient received CsA for 16 weeks. During this period, the CsA concentration was measured 31 times, with results from 98 μg/L to 219 μg/L (mean, 156 μg/L). Four weeks after transplantation, the patient developed a mild GvHD of the skin, which disappeared immediately after commencement of prednisolone treatment. Because B lymphocytes were absent or low early after transplantation, the patient received immunoglobulins for 5 months (Fig. 1). Endogenous immunoglobulin production started only in the later phase of immune reconstitution. Six weeks after discontinuation of CsA therapy, a concentration of 147 μg/L was still detected in whole blood. Although the patient was not supposed to have received CsA and the parents had denied the administration of CsA or drugs other than those prescribed, CsA concentrations between 116 μg/L and 174 μg/L were obtained over the next 4 months.
Questions to Consider
- Has the CsA therapy really bee discontinued?
- Can delayed drug elimination explain the continued increased CsA concentrations?
- What tests could be done to identify any potential analytical interference?
Final Publication and Comments
The final published version with discussion and comments from the experts appears
in the May 2011 issue of Clinical Chemistry, approximately 3-4 weeks after the Student Discussion is posted.
Educational Centers
If you are associated with an educational center and would like to receive the cases and
questions 3-4 weeks in advance of publication, please email [email protected].
AACC is pleased to allow free reproduction and distribution of this Clinical Case
Study for personal or classroom discussion use. When photocopying, please make sure
the DOI and copyright notice appear on each copy.
DOI: 10.1373/clinchem.2010.148718
Copyright © 2011 American Association for Clinical Chemistry