Welcome to the inaugural edition of Laboratory Stewardship Focus, a new section of Clinical Laboratory News that will appear quarterly.
This section follows a successful decade of CLN Patient Safety Focus, which ran quarterly from July 2008 to July 2018. Over those years, Patient Safety Focus benefited greatly from contributing authors from institutions across the United States, and covered topics not commonly found in the peer-reviewed or popular laboratory literature.
These included neglected areas such as the role of fatigue in errors, the competence-confidence conundrum, apology for errors, response to large-scale lab errors, failure to retrieve results, management approaches to improving a struggling lab, and more. This content remains archived on the AACC website or available by request.
Over the last decade, patient safety has become a mainstream topic. Patient safety programs are embedded in most health systems in the U.S., and most clinical laboratorians have access to copious resources about the nexus between laboratories and patient safety.
At the same time, a new domain in medicine—laboratory stewardship—is growing in importance but still neglected relative to other aspects of laboratory quality. Laboratory stewardship refers to correctly ordering, retrieving, and interpreting laboratory tests. Errors in these three aspects of the total testing process cause most diagnostic errors and the majority of significant patient harm and malpractice litigation in the laboratory industry. In addition, laboratory stewardship encompasses fair payment to labs and reasonable insurance coverage for medically necessary testing for patients. Thus, laboratory stewardship is where patient safety meets financial responsibility. It is where laboratory leaders strive to align with doctors, patients, and payers rather than conflict with them.
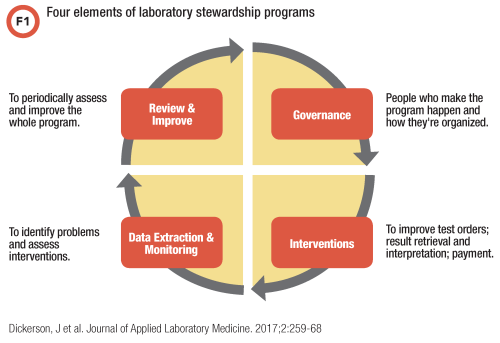
As shown in Figure 1, the four components of a laboratory stewardship program are: 1) governance, usually through a combination of permanent and ad hoc committees; 2) interventions to improve test ordering, retrieval, interpretation, and payment; 3) data to support all program elements; and 4) methods for periodically assessing and improving the program. In addition, stewardship programs partner with payers to develop evidence-based medical policies and reduce barriers to those policies through reasonable administrative rules that laboratory administrators and the average patient can traverse.
The new CLN Laboratory Stewardship Focus is supported by PLUGS (Patient-Centered Utilization Guidance Service), a grassroots organization of 99 member labs—including hospital-based and freestanding, for profit and nonprofit—and multiple sponsors who have created a network to share best practices in laboratory stewardship. PLUGS seeks to improve laboratory stewardship through nurturing this network, and accomplishes this by providing members with tools to stand up stewardship programs, by convening meetings to share best practices, and by forging collaborations directly with payers.
The philosophy of PLUGS is that most healthcare workers come to work to do a great job, whether they labor in a lab, in a clinic, or for a payer. Patients come to care facilities to interact with doctors, nurses, therapists, laboratory workers, and pharmacists. They expect lab services to be evidence-based and a covered benefit by their payer, and they want their laboratory care team to support their health while protecting them financially.
The editors of Laboratory Stewardship Focus welcome you to this experiment in stewardship education. We are a diverse group of editors consisting of a clinical chemist, an informaticist, two pathologists, and a genetic counselor. Our goal is to cover all aspects of laboratory stewardship using a variety of engaging formats including interviews, articles, literature reviews, cases, and more.
Welcome and feel free to submit your ideas for topics to www.seattlechildrenslab.org/plugs or any of the editorial board members.
Michael Astion, MD, PhD, is clinical professor of laboratory medicine at the University of Washington department of laboratory medicine and medical director of the department of laboratories at Seattle Children’s Hospital. Email: [email protected]
Jane Dickerson, PhD, DABCC, is clinical associate professor at the University of Washington and co-director for clinical chemistry at Seattle Children’s Hospital in Seattle. Email: [email protected]
References
- Dickerson JA, Fletcher AH, Procop G, et al. Transforming laboratory utilization review into laboratory stewardship: Guidelines by the PLUGS National Committee for Laboratory Stewardship. J Appl Lab Med 2017;2:259–68.
- Laposata M. The definition and scope of diagnostic error in the U.S. and how diagnostic error is enabled. J Appl Lab Med 2018;3:128–31.
- PLUGS. http://www.seattlechildrens
lab.org/plugs.aspx. (Accessed November 1, 2018).
- Dickerson JA, Cole B, Conta JH, et al. Improving the value of costly genetic reference laboratory testing with active utilization management. Arch Pathol Lab Med 2014;138:110–3.
- Riley JD, Procop GW, Kottke-Marchant K, et al. Improving molecular genetic test utilization through order restriction, test review, and guidance. J Mol Diagn 2015;225–9.
- Miller CE, Krautscheid P, Baldwin EE, et al. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A 2014;164:1094–101.
CLN's Laboratory Stewardship Focus is supported by Seattle Children's Patient-Centered Laboratory Utilization Guidance Services
