
The cellular phone, which recently marked its 40-year anniversary, has evolved from a 2 ½-pound, brick-like device to a palm-sized, 4-ounce mobile computer more powerful than most early desktop machines. Today, the cellular networks that power smartphones have become so pervasive and so powerful that mobile Internet usage is ready to surpass desktop usage. With smartphones and other mobile devices connecting wirelessly to almost anything digital, an explosion of mobile-capable devices is rapidly emerging as a billion-dollar industry.
A study published recently in Clinical Chemistry gives a glimpse into this future—powerful, miniaturized instruments paired with cellular networks and cloud computing that work even in remote parts of the world (Clin Chem 2013;59:629–40). In the study, researchers demonstrated that their rugged, credit card-sized microfluidic chip, called the mChip, could perform lab-quality HIV tests in Rwanda. In this case mobile means more than just small: the mChip device automatically synced results with patients' remote electronic health records (EHR) over cellular networks—all at lower cost and in less time than bench-top equipment laboratorians use every day in modern, sophisticated labs.
The marriage of diagnostics and mobile connectivity means more than just a new stream of gadgets, according to cardiologist, geneticist, and mobile health expert Eric Topol, MD. "I actually think this is the next big wave of where the diagnostics field will go," Topol said. "It's basically an outgrowth of the remarkable progress that's been made in microfluidics. For example, if you connect lab-on-a-chip hardware to a smartphone, that smartphone can do a lot of the processing, display, and archiving of the data. Already the likes of thyroid function tests, liver tests, and electrolytes are in the pipeline. This is definitely happening." Topol is professor of genomics and the director of the Scripps Translational Science Institute in La Jolla, Calif. He previously chaired the department of cardiovascular medicine at Cleveland Clinic, and last year was voted the Number One Most Influential Physician Executive in Healthcare by Modern Healthcare.
Tiny Instruments, Big Impact
The lab-on-a-chip concept has fueled predictions about mobile diagnostics for years. But few of these devices have proven successful in practice. According to mChip designer Samuel Sia, PhD, lead author of the study in Clinical Chemistry, researchers have to look beyond pure analytical performance. "You can have the best performing technology in the world, but if it requires too many steps by the user or complicated equipment, it will never be used in the field," he said. "One really needs to think about how to integrate every component from the beginning: fluid handling, signal amplification and detection, data communication, as well as user experience—all of this has to work together seamlessly." Sia is an associate professor of biomedical engineering at Columbia University in New York City and co-founder of Claros Diagnostics, now called OPKO Diagnostics.
To be sure, the mChip is a workhorse. The device captures all the essential functions of an ELISA in an elegant, injection-molded translucent plastic card, with reagents tucked away inside a nearly invisible lattice of precision channels (See Photo, below). A compact reader initiates the test and displays results with the push of a single button. In the Clinical Chemistry study, the mChip demonstrated 100% sensitivity and specificity for HIV using 1 µL of whole blood from a finger prick, outperforming traditional lateral-flow rapid tests and matching bench top ELISA performance in under 15 minutes. Importantly, the mChip also correctly identified weakly positive samples, the Achilles heel of many rapid HIV tests based on traditional technology.
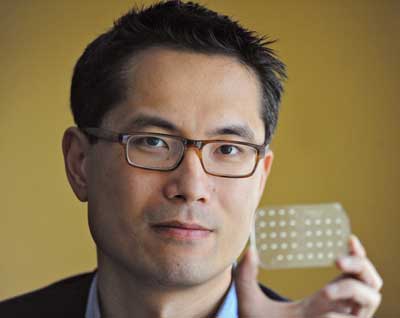
Samuel Sia, PhD, holds his mChip device, a microfluidic card
that can perform up to 10 lab-quality ELISA assays in less than 15 minutes.
Courtesy of Eileen Barroso for Columbia Engineering
However, Sia and his colleagues didn't stop there. They incorporated two wireless communications technologies into the compact mChip reader: a satellite transceiver for global coverage and another for local cellphone towers. This way, results automatically became part of patients' EHRs, along with a date and location stamp, no matter where or when the test was performed. Such a mobile uplink to EHRs enables healthcare workers to monitor outbreaks of disease, strategically allocate medications and other resources, begin treatment faster in remote settings, and reduce the errors inherent in manual transcription of results, according to the researchers.
Although the compact reader for the mChip displays results clearly to the user, Sia's team had a broader clinical need in mind when they integrated cellular connectivity. "We wanted to build a real product, not just show proof of concept, and if you want to build a product that people will use, it's important to consider what the clinical need actually is," Sia explained. "In this case, our aim is to improve care for pregnant women in remote settings in the developing world. When a woman comes in for testing, it's vital that the healthcare worker know the patient's history, so whatever testing they perform needs to be linked to both past and future patient records. That's why we designed the device to automatically sync with the patient's electronic record."
Engineering for Cost and Simplicity
Currently available rapid tests, such as lateral flow-based HIV screening kits now available over the counter, often rely on pushing the limits of traditional methods in order to muster the requisite sensitivity and specificity. The emerging microfluidic methods exemplified by the mChip have to move in the opposite direction: they start with high performance, but their intricate design can threaten usability and reliability. For microfluidics to become part of useful, mobile devices, engineers face an uphill climb, Sia said.
"Our approach is to take a platform that is intrinsically high performing, and then try to reduce its complexity so that it can be affordable and usable in the field," Sia said. "Affordability and usability are not just an afterthought. You can't single-mindedly pursue performance, and expect only later on to consider making all the steps automated. That kind of thinking doesn't lead to a wholly integrated device that works well for the user." Sia believes his research team can get the cost down to about $1 per test for the mChip, compared to $1.80–$6.20 for a standard bench top ELISA. The total cost of the mChip system is about $1,000 versus $20,000.
Topol, who recently published a book, The Creative Destruction of Medicine: How the Digital Revolution Will Create Better Healthcare, has kept a close eye on mobile health companies. Last year, he co-authored an opinion article in Clinical Chemistry enumerating a handful of cutting-edge technologies he believes signal the advent of "unplugged laboratory medicine" (Clin Chem 2012;58:1644–7). "By using a customizable array of sensors, reagents, and substrates, microfluidics devices serve as miniature factories that ‘shrink the plumbing' typically found in traditional laboratories into a palm-size device. The advantages are substantial: speed, portability, and capability to perform a wide array of biochemical testing," Topol wrote. "Integration with smartphones will ultimately help emerging lab-on-a-chip devices bridge the existing gap between bioengineering prototypes and commercially viable clinical tools. By providing mobile access to computing power and Internet connectivity, smartphones also offer sensors, cameras, and high-resolution displays that can be used to visualize data and enable telemedicine applications."
However, for those mobile health technologies that do prove themselves effective, cost remains a big hurdle, Topol told CLN. "One of the biggest problems that we always confront at the intersection of technology and medicine is: will it reduce costs? There is a long history of new, innovative technology coming in and actually raising costs," he said. "That has to be resolved."
It's hard not to compare lab-on-a-chip devices to the computing hardware that's become commonplace in modern life. Computers that once filled entire rooms have rapidly shrunk in size and increased in power to the point that computing power is mostly invisible, encapsulated in tiny microprocessors hiding inside smartphones, televisions, and toys. In some ways, the lab-on-a-chip arena has followed a narrative similar to the computing evolution in Silicon Valley, taking each step of performing an assay and making it progressively smaller and faster.
According to Sia, lab-on-a-chip microfluidics is certain to evolve, but lab-quality test results on mass-market, consumer-oriented devices will take time. "I think the time scale is still an open question, in terms of when we'll get to that point where people can diagnose a range of conditions on their smartphone," he said. "I think that point will happen. In fact it's probably inevitable that it will happen. But if we were to push the analogy, I think where we are now with diagnostics is probably comparable to the early 1980s of computing. Most diagnostic tests are still run in centralized labs, and the question is, can we miniaturize these tests to the point where the everyday consumer can really use them. If people think putting together an iPhone is hard, putting together one of these diagnostic mobile health devices is at least an order of magnitude harder, because you have to integrate so many things together—not only software and hardware, but the chemistry and biology that are notoriously fickle."
Liberating Data
Microfluidic technology will no doubt continue to fascinate and inspire engineers, laboratorians, and diagnostics companies. However, mobile health is about more than just new diagnostic technology. Familiar instruments can take on new power when they incorporate mobile connectivity. Take the humble blood glucose meter used by millions of people with diabetes. Last year, Bethesda, Md.-based Telcare, Inc. launched the first-of-its-kind cellular-enabled meter that automatically uploads results to an online portal—no smartphone required. With the data in the cloud, patients can provide their caregivers secured access to customized graphs and reports to make sense of the patients' myriad of daily test results.
With nearly 30,000 units sold, Telcare has branded its wireless meter as both a patient engagement device and a population health management tool. Patients can view serial reports about their glucose readings online or on companion smartphone apps (See Screen Image, below). They also can receive customized education and other messages based on their test results, right on their meter.
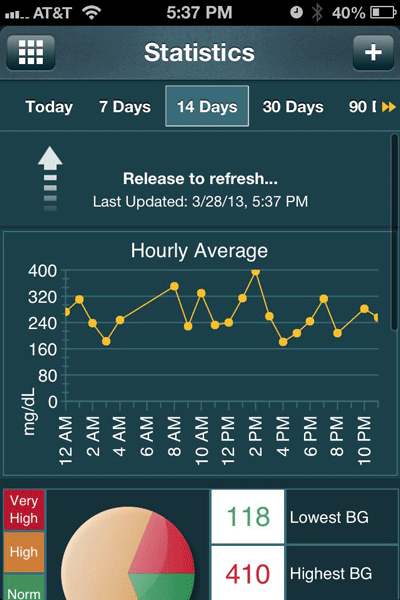
A companion iPhone app neatly graphs data
from the wireless Telcare blood glucose meter.
The meter automatically uploads test results for analysis
via an online portal by physicians or other caregivers authorized by patients.
Courtesy Telcare, Inc.
According to David Bjork, Telcare's president and COO, liberating the data from blood glucose meters opens up a host of new uses for test results that otherwise would remain hidden or forgotten on conventional meters. "The whole notion is about making use of this data to drive patient engagement, which in turn motivates patients to be more self-aware and more educated," Bjork said. "We can also observe this data and then do something with the observation in order to assist that person. We can even alert a healthcare professional when a patient is struggling and not making wise decisions."
This can make a big difference for patients living with diabetes who many times feel abandoned after an initial diagnosis to manage their own care, Bjork said. "Those patients who are very ill do receive attention from the healthcare system, but that's only about ten percent of those living with the disease," he said. "The majority of people with diabetes are basically on their own—until they require expensive interventions. Our healthcare system doesn't do a good job right now of managing the entire population of people living with diabetes."
With a new cache of patient data in hand, Telcare is also building bridges to providers' EHRs, making test results that once died on patients' meters at home part of the same records available in hospitals or doctors' offices. While the company remains focused on diabetes, how successful this integration becomes could bode well for other mobile testing platforms.
Physicians, especially endocrinologists, are beginning to use the Telcare portal. But so far the greatest interest has been from payers and other risk-bearing organizations, such as self-insured employers, commercial insurance companies, and accountable care organizations (ACO), Bjork noted. Telcare is working to analyze the data points from thousands of wireless meter results and pinpoint individuals most at risk. Payers see the potential here to identify which patients need extra help before more costly interventions are required, saving the system money in the long run.
"In some respects, the integration has been more intense on the payer side. These payer organizations that bear financial risk for populations find the information we're collecting very valuable," Bjork said. "An organization that bears financial risk is much more apt to stand in the front of the line and say, I want to deploy this platform because it can change my risk profile. If you can observe patient behavior, then you can identify risks in the population and start proactively supporting people that are otherwise managing their conditions by themselves."
ACOs, value-based contracts among insurers and providers, and similar reimbursement models that emphasize paying for value versus fee-for-service drive adoption, according to Bjork. "The minute that this type of contract is in place, our platform becomes very attractive, because physicians now have an incentive to pay attention to patients between office visits. With our platform, they can monitor that population of patients for whom they now carry a financial risk."
| To hear Eric Topol, MD, describe the new opportunities that exist with mobile technologies and how these innovations will change the future of laboratory testing in a podcast from Clinical Chemistry, go online. |
A Consumer-Driven Market
Of course, most mobile health apps and devices don't have a long history inside the healthcare system like a blood glucose meter. Companies seeking to break into mobile health from the outside will have to focus on appealing directly to consumers.
A recent mobile health market report from Berlin-based firm research2guidance predicts the market will grow to $26 billion within 5 years (See Box, below). However, most of this growth will likely not come from Food and Drug Administration (FDA)-cleared products covered by insurance such as Telcare's blood glucose meters. Rather, companies will depend on consumers dipping into their own pockets, according to Ralf-Gordon Jahns, cofounder of research2guidance. "A consumer-driven market means that an app or companion device will have to be so attractive and so useful to patients that they are willing to pay for it themselves," he said. "That means that you have to have a more consumerist design, with a product that is very easy to use and understand. For at least the next few years, companies simply can't rely on most payers in the healthcare system to be quick to adopt mobile health solutions."
|
Mobile Health Market Poised for Growth
FDA Vows Not to Suppress Innovation in New Regulations
According to experts, the mobile health industry is still in the early stages of a growth curve. A report published in March from the Berlin-based mobile market research firm research2guidance estimates that 500 million smartphone users worldwide will be using a healthcare app by 2015. The report predicts that in 5 years, the so-called mHealth market will achieve "mass market status," with 50% of the estimated 3.4 billion smartphone users having downloaded mHealth applications. In the 2013–2017 period, the firm forecasts market revenue growth of 61%, reaching $26 billion. To put this number in perspective, that's nearly a third of what market researchers expect the in vitro diagnostics market to reach in the same timeframe, $80 billion.
As recently as 2011, the market for health-oriented mobile apps began to change, according to the report. Whereas previously the business model revolved around a pay-per-download model for smartphone apps, the ascending model has relied on services, companion devices, and in-app purchases. Now, free applications serve as platforms for mobile health services and hardware. For example, the iHealth blood pressure app that charts daily readings is free, but the accompanying blood pressure cuff and iPhone dock costs $72.99 in retail stores.
Taking the idea a step further, imaging and other devices that depend on mobile computing power have already hit the market, such as AliveCor's Food and Drug Administration (FDA)-cleared electrocardio-gram with a sensor that snaps to the back of an iPhone.
Pending regulations for mHealth from FDA will also play a role in driving growth. Ironically, the lack of regulation has actually hampered more rapid growth in health-related mobile devices, according to research2guidance co-founder Ralf-Gordon Jahns. "The uncertainty around regulation has stalled a lot of companies in the mHealth area," he said. "You can't build a solid business case around an mHealth device when you don't know what the FDA is going to require or which apps could be regulated as medical devices."
FDA released a draft guidance in 2011 covering how it would regulate health-related apps, but the agency left a lot of gray area that only served to increase anxiety for mHealth app and device developers. In a March 21, 2013 hearing convened by the House Energy and Commerce Committee, Christy Foreman, director of the FDA's device evaluation division, sought to assure Congress that the agency would not stifle innovation, and promised to release a final version of the long-awaited guidance in October. She emphasized that only apps that sought to perform functions of bona fide medical devices would come under scrutiny from the agency.
Once FDA acts, it could prove to be a turning point for the health-related sector of the mushrooming mobile market, Jahns said.
|
Topol emphasized that he believes many healthcare professionals underestimate consumers. He expects mobile health to become a positive feedback loop, in which increasingly empowered consumers demand more and more. "I feel that a lot of people don't respect the capability, desire, and motivation of patient consumers," Topol said. "My experience has been that when patients have access to their data, it's such a decided advantage. It switches people who would be the least likely to be data-driven to become data-driven."
In addition, now that consumers have an appetite for mobile health technology, social media have the ability to drive adoption much faster than in the past, Topol added. "We have a special situation today where new products can go viral very rapidly with social networking, so that a consumer has a much higher level of power to bring in other consumers than ever before. If this gets legs among some key parties, adoption of mobile health apps and devices could happen a lot faster compared to other products in the past."