
Osteoporosis, a word derived from the Greek for bone and pore, is a disease characterized by low bone mass and micro-architectural deterioration of bone tissue, leading to enhanced bone fragility and consequent increase in fracture risk. The disease is most common in women after menopause (primary type 1) and can worsen after age 75 (primary type 2); however, males are also affected by the disease.
Osteoporosis is a silent disease with no symptoms, but its clinical consequences can be debilitating. Common sites for osteoporotic fractures include the spine, wrist, and hip. The aging population, as well as lifestyle changes, have led to an increase in the disease's prevalence and the incidence of fractures. In fact, fractures account for the majority of osteoporosis-associated health costs, with hip fractures causing the most morbidity and mortality and accounting for a large proportion of the cost. In the U.S., the annual cost of osteoporotic fractures has risen from $17 billion in 2006 to approximately $20 billion today, and it is predicted to rise to $25 billion by 2025.
The World Health Organization (WHO) diagnostic criteria for osteoporosis includes a bone mineral density (BMD) measurement ≥2.5 standard deviations (SD) below the average value for young healthy women (T-score ≤ -2.5 SD). Osteopenia, marked by a borderline decrease in BMD, is defined as a BMD T-score between -1.0 and -2.5 that is based on the most widely validated technique to measure BMD, dual energy X-ray absorptiometry (DXA).
Bone turnover markers (BTMs) also hold promise for evaluating fracture risk and monitoring osteoporosis treatment. Recently, the International Osteoporosis Foundation (IOF) and International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) joined forces to create a Working Group on Bone Marker Standards (WG-BMS) to identify consensus reference standards. This article will provide an overview of the pathophysiology of osteoporosis and current clinical use of BTMs and describe the WG-BMS's standardization efforts.
Bone Remodeling
Even after growth is complete, the adult skeleton continues to be renewed by a process called remodeling that repairs areas of micro-cracks and helps prevent accumulation of fatigue damage. Remodeling, which is also important for the maintenance of calcium homeostasis, is a bone surface phenomenon that occurs in discrete units, called bone remodeling units. Initially, osteoclasts, a type of bone cell that removes the mineralized matrix, attach to the site of remodeling and excavate an erosion cavity. When this phase is complete, osteoblasts responsible for bone formation migrate to the newly resorbed cavity, lay down a new matrix of osteoid—composed mainly of Type I collagen—and contribute to mineralization of the bone. In a steady state, the amount of new bone formed is equal to the amount resorbed.
Osteoporosis occurs if there is an imbalance in the amount of bone formed and resorbed over time. This occurs when there is either an increase in the amount of bone resorbed and/or a decrease in the amount of new bone formed. In addition, when the rate of formation of new resorption cavities increases, bone loss accelerates.
Bone Turnover Markers
There are two types of BTMs: enzymes secreted by osteoclasts and osteoblasts, and structural proteins or their fragments either secreted by osteoblasts during bone formation or released by degradation of the collagen matrix of bone during bone resorption. These markers circulate in blood and/or are excreted in urine, and measuring them provides a quantitative estimate of the current rate of bone remodeling or turnover.
However, the accuracy with which BTMs reflect the rate of bone remodeling may be influenced by a number of physiological and pathological factors. Although BTMs are classified as either formation or resorption markers, to some extent, they reflect both bone formation and resorption. Generally, bone formation and resorption are coupled. When resorption is increased, so is formation. Similarly, when the former is decreased, the latter follows suit. Exceptions do occur, however. As an example, increased bone resorption with suppressed bone formation occurs in patients on glucocorticoid therapy and in those with systematic inflammatory disease or multiple myeloma, as well as in immobilized individuals. While the newer BTMs are reasonably specific to bone tissue, contribution from other tissues cannot be totally excluded.
In women, bone remodeling increases at menopause due to estrogen deficiency and remains elevated as women age. This imbalance increases the risk of fractures, which may be partly due to the decrease in BMD; however, the effect of increased bone remodeling on fracture risk is also independent of BMD. Deterioration of bone architecture may contribute to bone fragility over and above that caused by the decrease in BMD. In addition, increased bone turnover increases the proportion of newly formed bone, which is less well-mineralized than mature bone and has fewer post-translational modifications of bone collagen, such as cross-linking and ß-isomerization. Consequently, measuring women's BTMs may offer information in addition to BMD for identifying patients at increased risk of fracture and as candidates for treatment.
The majority of minimal trauma fractures occur in individuals with osteopenia and normal BMD because of their higher prevalence. Age, sex, a history of prior fractures, parental hip fracture history, body mass index, ethnicity, smoking, alcohol use, glucocorticoid use, and rheumatoid arthritis are also independent contributors to fracture risk.
To help clinicians evaluate patients' fracture risk and identify those who might benefit from anti-osteoporosis therapy, WHO has developed the FRAX tool (FRAX). This algorithm takes into account the contribution of each of the risk factors listed above, except BTMs, which were excluded due to the lack of adequate data from population-based prospective studies. For the same reason, most osteoporosis guidelines do not recommend BTMs to assess patients for disease.
On the other hand, there is good evidence for monitoring osteoporosis treatment with BTMs, especially with the most widely used anti-resorptive agents. In fact, BTMs show large and rapid responses in this clinical setting. After beginning therapy, patients have an early decrease in bone resorption markers followed by a decrease in bone formation markers due to the coupling effect (Figure 1, below). Conversely, treatment with an anabolic agent such as teriparatide leads to an increase in bone formation markers followed by an increase in bone resorption markers. The speed and magnitude of these changes following treatment contrasts sharply with the slow and small changes seen in patients' BMD.
These observations suggest that measuring patients' BTMs 3–6 months after starting therapy may be useful for assessing how well it is working. Guidelines recommend measuring BMD 18–24 months after patients start treatment; however, this is a long time to wait to confirm treatment failure or success. Furthermore, change in BTMs explains a greater percentage of the fracture risk reduction than does change in BMD. Clearly, more data are needed on individual BTMs in terms of their ability to confirm treatment effect, predict fracture risk reduction, and improve compliance with treatment.
Figure 1
Changes in Bone Markers Following Treatment
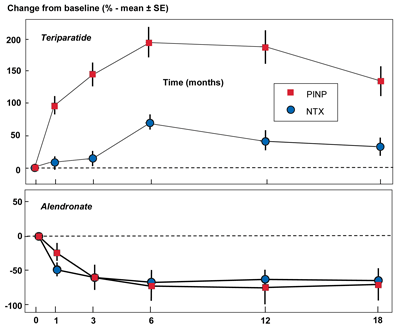 The graphs show the mean percentage change plus or minus the standard error of the mean in two bone markers, NTX (bone resorption) and PINP (bone formation), as a percentage of baseline level over an 18 month period following initiation of treatment. The upper panel is following treatment with an anabolic therapy (teriparatide) and the lower panel is following treatment with an anti-resorptive therapy (alendronate).
Used with permission from Vasikaran S et al. Osteoporos Int 2011;22:391–420.
The graphs show the mean percentage change plus or minus the standard error of the mean in two bone markers, NTX (bone resorption) and PINP (bone formation), as a percentage of baseline level over an 18 month period following initiation of treatment. The upper panel is following treatment with an anabolic therapy (teriparatide) and the lower panel is following treatment with an anti-resorptive therapy (alendronate).
Used with permission from Vasikaran S et al. Osteoporos Int 2011;22:391–420.
BTMs in Clinical Use
Over the last few decades, researchers have studied a number of BTMs for assessing osteoporosis, as well as other bone diseases. Among these markers, two formation markers (serum bone-specific alkaline phosphatase (s-BALP) and serum procollagen type I N-terminal propeptide (s-PINP)) and two bone resorption markers (urine N-terminal telopeptide of type I collagen (u-NTX) and serum C-terminal telopeptide of type I collagen (s-ßCTX)) are the most commonly used in current clinical practice (Table 1, below). Commercially available immunoassays for all the markers are reasonably bone-specific and reflect bone turnover in postmenopausal osteoporosis and following anti-resorptive therapy. However, their levels may be influenced by time of collection and fasting status. In order to obtain meaningful results, it is important to minimize these effects by standardizing collection protocols.
|
Table 1
Bone Turnover Markers in Clinical Use
|
| Marker |
Origin |
Comments |
| s-BALP |
Ubiquitous, membrane-bound tetrameric enzyme located on the outer cell surface of osteoblasts |
Specific for bone, but with some cross-reactivity with liver isoform (up to 10%).
Variability: very small circadian rhythms.
|
| s-PINP |
Precursor molecules of collagen type I synthesized by osteoblasts (and fibroblasts) |
Mostly derived from bone collagen type I.
Assay: May recognize trimer alone (intact), or trimer plus monomer (total PINP).
Variability: small circadian rhythm.
|
| u-NTX |
Osteoclastic hydrolysis of collagen type I |
Needs to be corrected for urinary creatinine.
Collagen type I, with highest contribution probably from bone.
Variability: significant circadian rhythm.
|
| s-ßCTX |
Osteoclastic hydrolysis of collagen, generated by cathepsin K |
s-CTX is always ß-isomerized.
Specificity: collagen type I, with highest contribution probably from bone.
Variability: Very dependent on time of day and food (must be collected after an overnight fast); influenced by renal function, liver function, and circadian rhythm.
|
| Abbreviations: s-BALP, serum bone-specific alkaline phosphatase; s-PINP, serum procollagen type I N-terminal propeptide; u-NTX, urine N-terminal telopeptide of type I collagen; and s-ßCTX, serum C-terminal telopeptide of type I collagen. |
BTM Reference Standards
As mentioned previously, clinicians traditionally have relied on assessment of BMD to identify patients who should be treated for osteoporosis. Recognizing the need to accumulate adequate information on BTMs' utility for fracture risk, the WG-BMS formed to review the published literature and identify potential candidates as markers of bone resorption and bone formation. We reviewed population-based studies that used currently available osteoporosis treatments and concluded that our efforts should be focused on a single bone formation marker and a single bone resorption marker.
While acknowledging the lack of a gold standard BTM, the working group recommended that s-PINP and s-ßCTX be designated as reference standard markers of bone formation and resorption, respectively (Table 2, below). As such, we also recommended that these two markers be included in all clinical trials and in observational studies in order to accumulate data for their application in clinical practice. This recommendation does not preclude using other BTMs in osteoporosis studies; instead it provides internal references and the ability to compare studies more easily.
In the U.S., the National Bone Health Alliance (NBHA) has published a position paper endorsing the choice of s-ßCTX and s-PINP as the reference standards for bone resorption and formation, respectively, in osteoporosis.
The WG-BMS has also identified several areas that should be carefully controlled when studying the reference standard BTMs in future clinical trials, including handling samples appropriately, measuring the markers in all patients in clinical trials, and using appropriate statistical methods for results analysis. The group has also developed some key questions: 1) Do BTMs predict fractures, and if so, what is the nature of that relationship? 2) Can BTMs be considered alongside other risk factors for fracture in absolute fracture risk algorithms such as FRAX? 3) in clinical trials of osteoporosis treatment, what is the relationship between change seen in BTMs following treatment and fracture risk reduction?
Other possible uses of BTMs that also need further study include: predicting rate of bone loss, identifying secondary osteoporosis, targeting interventions, and improving patient compliance. Finally, characterization of reference intervals for relevant populations remains a top priority.
|
Table 2
Criteria for Selection of Reference Bone Turnover Marker
The International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine Working Group on Bone Marker Standards used the following criteria to select a reference bone turnover marker.
The marker must:
- Be adequately characterized and clearly defined.
- Be bone specific.
- Perform well in fracture risk prediction, as well as in monitoring treatments used or in clinical trials, for osteoporosis treatment in both women and men.
- Be measurable by widely used methodology in automated clinical laboratories and not the intellectual property of a single manufacturer.
- Have biological and physico-chemical characteristics suitable for practical laboratory use in terms of biological and analytical variability, sample handling, stability, ease of analysis, etc.
- Be measureable in blood, as intra-individual variation is significantly less than for urine.
|
The Need for Assay Standardization/Harmonization
Significant inter-method differences exist for both s-ßCTX and for s-PINP, which makes it difficult to compare an individual's results generated in different laboratories. Furthermore, not only are these differences especially problematic for multi-center clinical trials, but they also make it impossible for treatment guidelines to designate universally applicable cut-points and decision levels.
A good example of these inter-method differences is reflected in the commercially available assays for s-ßCTX, all of which measure the same molecule. However, results of the United Kingdom National External Quality Assessment Service and other studies using stored samples have revealed some inter-assay biases. Better agreement may be obtained, however, when fresh samples are used. If all s-ßCTX assays measure the same peptide molecule, any inter-assay biases may be explained by differences in calibration of these assays and may be amenable to harmonization or standardization.
As stated in the position paper on BTM standards, IFCC and IOF aim through the WG-Standardization of Bone Marker Assays (SBMA) to standardize or harmonize, as appropriate, commercial assays for s-ßCTX and s-PINP. In the U.S., NBHA has also focused its efforts on harmonizing assays for the two BTMs, standardizing BTM sample collection procedures, and establishing reference intervals for the two reference BTMs in clinical use. These steps are all designed to help clinicians gain confidence in using BTMs to monitor patients' osteoporosis treatment and assess their future fracture risk.
The Future of BTMs in Patient Care
The goal of the WG-SBMA's efforts under the leadership of IOF and IFCC is to include evaluation of BTMs in fracture-risk algorithms and clinical guidelines together with evidence-based decision limits and treatment targets. These milestones are intended to help clinicians make optimum use of these markers in patient care. As described here, the group has set a clear path that it hopes will help focus research efforts in the future using these markers, and lead to acceptance of s-PINP and s-ßCTX as the reference standard markers for bone formation and degradation, respectively.
SUGGESTED READINGS
Bauer D, Krege J, Lane N, et al. National Bone Health Alliance Bone Turnover Marker Project: Current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int 2012;23:2425–33.
Chubb SA. Measurement of C-terminal telopeptide of type I collagen (CTX) in serum. Clin Biochem 2012;45:928–35.
Eastell R, Garnero P, Audebert C, et al. Reference intervals of bone turnover markers in healthy premenopausal women: Results from a cross-sectional European study. Bone 2012;50:1141–7.
Koivula MK, Risteli L, Risteli J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin Biochem 2012;45:920–7.
Lombardi G, Lanteri P, Colombini A, et al. Blood biochemical markers of bone turnover: Pre-analytical and technical aspects of sample collection and handling. Clin Chem Lab Med 2012;50:771–89.
McCloskey E, Johansson H, Oden A, et al. Fracture risk assessment. Clin Biochem 2012;45:887–93.
Miller WG, Myers GL, Gantzer LM, et al. Roadmap for harmonization of clinical laboratory measurement procedures. Clin Chem 2011;57:1108–17.
Schousboe JT, Bauer DC. Clinical use of bone turnover markers to monitor pharmacologic fracture prevention therapy. Curr Osteoporos Rep 2012;10:56–63.
Vasikaran SD. The utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci 2008;45:221–58.
Vasikaran S, Eastell R, Bruyère O, et al.; IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos Int 2011;22:391–420.
 Samuel D. Vasikaran, MD, FRCPA, is head of clinical biochemistry, PathWest-Royal Perth Hospital and Fiona Stanley Hospital Network, Perth, Australia, visiting consultant, Osteoporosis Clinic, Royal Perth Hospital, and clinical professor, University of Western Australia. E-mail: [email protected]
Samuel D. Vasikaran, MD, FRCPA, is head of clinical biochemistry, PathWest-Royal Perth Hospital and Fiona Stanley Hospital Network, Perth, Australia, visiting consultant, Osteoporosis Clinic, Royal Perth Hospital, and clinical professor, University of Western Australia. E-mail: [email protected]