Cardiac troponins (cTn) have been available for nearly 2 decades in clinical laboratories and are now considered the gold standard for biochemical detection of myocardial infarction (MI). Furthermore, multiple organizations have endorsed the biomarkers’ use in both clinical and analytical guidelines (1, 2). Today, the universal definition of MI includes the typical rise and/or fall of cTn with at least one value above the 99th percentile of a healthy reference population accompanied by at least one of the following clinical factors: presence of ischemic symptoms; electrocardiographic changes; or imaging evidence of loss of viable myocardium or a new wall motion abnormality (3–6).
However, a growing body of evidence now suggests that very low cTn values are clinically important. Investigators have found that patients who have cTn elevations considered normal, but near the 99th percentile, have worse prognoses and require more aggressive clinical management. These findings have prompted the search for newer techniques to enhance precision and enable measurements of cTn at or even below the 99th percentile cutoff (7–10).
Recently, researchers and commercial manufacturers have developed several high-sensitivity assays for cardiac troponin (hs-cTn) that are expected to be available soon for routine clinical use in the U.S. (11–15). Understanding their analytical and clinical performance will be extremely important because under the current MI definition, a significant proportion of the general population would have evidence of myocardial injury (16, 17). In this article, we will review the basic analytical and clinical characteristics of hs-cTn assays that are important for laboratory professionals to understand and describe how best to help clinicians employ these powerful assays.
Analytical Considerations
In order to be designated high-sensitivity, cTn assays must demonstrate a clear technological advancement over earlier generation assays. In other words, hs assays should measure cTn well below the 99th percentile cutoff with an acceptable coefficient of variation (CV). Given their enhanced analytical sensitivity, these assays have the potential for significant pre-analytical issues. For instance, even mild degrees of hemolysis can reduce hs-cTnT values (18). Researchers have found that sample type also affects results (19) and may impact clinical interpretation (20). Other clinical conditions such as skeletal muscle disease have been found to affect cTnT concentrations (21), and accumulating evidence for certain assays suggests there are sex-specific differences in the 99th percentile upper limits (22).
As researchers evaluate the new hs-cTn assays being developed by diagnostic manufacturers, data are emerging on their clinical properties. Below we summarize some of the important characteristics that laboratorians need to be aware of before implementing these tests.
Figure 1
Comparison of 19 Cardiac Troponin Assays
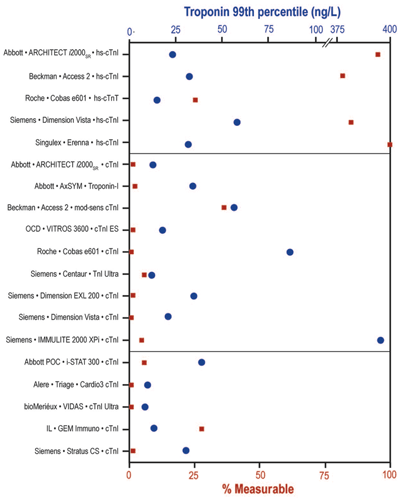 Comparison of both 99th percentile values (circles) and percent measurable concentrations (boxes) in a presumably healthy population for 19 cardiac troponin assays designated by hs (top section), sensitive-contemporary (middle section), and POC (bottom section); mod-sens, modified-sensitive.
Reprinted with permission of Clinical Chemistry from reference 29.
Comparison of both 99th percentile values (circles) and percent measurable concentrations (boxes) in a presumably healthy population for 19 cardiac troponin assays designated by hs (top section), sensitive-contemporary (middle section), and POC (bottom section); mod-sens, modified-sensitive.
Reprinted with permission of Clinical Chemistry from reference 29.
Beckman Coulter hs-cTnI Assay
Investigators recently evaluated the analytical and clinical performance of Beckman Coulter’s prototype hs-cTnI assay on patients from the Global Utilization of Strategies to Open the Occluded Arteries-IV trial (GUSTO-IV) (13). The cohort included 542 healthy subjects and 1,503 acute coronary syndrome (ACS) patients with either unstable angina and/or non-ST elevation MI. The assay detected cTnI in >95% of healthy subjects, with a median level of 3.2 ng/L and a 99th percentile value of 10 ng/L. This assay also showed good discrimination between healthy subjects and ACS patients at a level of 6.4 ng/L with a sensitivity and specificity of 84.8% and 89.7%, respectively. No significant differences between men and women were found at the 99th percentile cutoff; however, individuals with cTn levels above this cutoff had significantly higher mortality and MI rates compared to those with levels below the cutoff. Based on this cohort, the investigators concluded that the assay was helpful for diagnosing patients with suspected myocardial ischemia and for assessing their prognosis (13).
Another recent study extended these findings to the high-risk, stable population. Investigators working on the Heart Outcomes Prevention Evaluation study (HOPE) also confirmed that cTnI levels >6 ng/L at a ≤99th percentile cutoff of 10 ng/L were predictive of future MI and cardiovascular death (23).
Singulex hs-cTnI Assay
Investigations of the Singulex prototype hs-cTnI assay have focused primarily on healthy volunteers (24). In this population, researchers found that cTnI concentrations were normally distributed with a 99th percentile cutoff of 7 ng/L. The study also included a limited comparison of 15 samples from three acute MI patients. Using the hs assay, the serial measurements became positive earlier for the initial admission sample with a sensitivity of 53% compared to the standard sensitivity cTnI assay with 0% sensitivity.
The investigators also used the hs-cTnI assay to evaluate 50 non-MI chest pain cases, all of whom had undetectable troponin levels using the normal assay. In this population, cTnI levels in approximately 25% of patients exceeded the 7 ng/L cut-off. The investigators speculated that these patients may have had minor myocardial damage; however, the clinical significance of this finding is not known because outcome data were not pursued. Another recently published study of individuals in the population-based Minnesota Heart Survey also demonstrated that this high-sensitivity assay predicts those at highest risk for cardiovascular death (25).
Roche Diagnostics hs-cTnT Assay
The assay with the largest number of published studies, including both acute and stable populations, is the Roche hs-cTnT assay. In the ACS setting, this hs-cTnT assay was clearly superior to the fourth-generation assay, with a clinical performance similar to sensitive cTnI assays (26). However, researchers are not in agreement about how analytically sensitive the hs-cTnT assay is compared to hs-cTnI assays. Current studies comparing hs-cTn assays in various settings are aimed at settling this debate (27).
Siemens hs-cTnI Assay
A study of 304 healthy subjects using the Siemens prototype hs-cTnI assay found normally distributed values with a 99th percentile cutoff of 9 ng/L (28). Researchers also evaluated the assay’s clinical utility for diagnosing MI in 653 patients from the PROBE-ACS study (28), a patient cohort presenting with suspected ACS. They used the Siemens ADVIA Centaur Ultra-TnI assay to diagnose MI and then compared the results. The Ultra-TnI assay’s sensitivity and specificity for diagnosing MI were 94% and 81%, respectively, while the hs-TnI assay had a sensitivity of 93.7% and specificity of 91.1%. In another reference-interval study, the 99th percentile cutoff was reported to be 5.8 ng/L, highlighting the fact that not only this assay, but in fact the majority of hs-cTnI assays, need to be evaluated further (29).
Abbott hs-cTnI Assay
Abbott reports its hs-cTnI assay detects as little as 3.4 ng/L with a CV of 10% at 5.2 ng/L. In a study of 4,139 individuals from the population-based Gutenberg Health Study, investigators determined that 30 ng/L was the diagnostic cutoff representing the 99th percentile of a reference population. The study cohort contained 1,818 patients with suspected ACS who were consecutively enrolled at chest pain units across Germany. Of those patients, 23% were diagnosed with MI. The assay had a sensitivity of 82.3% and a 94.7% negative-predictive value for ruling out MI in patients evaluated at admission using a 99th percentile diagnostic cutoff value of 30 ng/L. On values obtained 3 hours after admission, the sensitivity was 98.2% and the negative-predictive value was 99.4% for both hs-TnI and cTnI assays (30).
Monitoring Delta cTn Values
A fundamental component of the universal MI definition is a typical rise and/or fall in troponin levels. However, to improve the specificity of cTn for diagnosing MI, some researchers have proposed using a delta cTn value. While there is consensus that the change used for interpreting acute MI must exceed both the biological and analytical variance of the measurements, determining the appropriate magnitude of this change has been an area of some controversy. Most studies that have looked at the delta criterion have used repeat cTn measurements between 1 and 6 hours after presentation (11, 31, 32); however, other acute cardiac conditions also can produce substantial temporal changes in troponin values (32).
Specific delta values are also controversial. Some researchers argue that delta values sacrifice diagnostic sensitivity for specificity, while others say the amount of cTn release is unpredictable and too complicated. Furthermore, the change in cTn values—whether an absolute number, percentage, or combination of both—will most likely be both assay- and time-dependent, making interpretation of the results extremely complicated and perhaps even more confusing.
Limitations of the Current Approach
Most studies evaluating the utility of cTn assays have focused on predetermined thresholds. Although commonly used, thresholds based upon 99th percentiles or CVs <10% are arbitrary. It is important to recognize that studies demonstrating worse prognosis above these thresholds do not confirm the point at which a patient’s risk is actually changing. Such results may be driven by the poor outcomes of patients with cTn measurements substantially above these thresholds. Furthermore, some patients with cTn values immediately below the thresholds may also have poor outcomes, but such data points do not stand out in the larger patient population with even lower cTn values and few or no events. For these reasons, there is a need for a large study with more than 10,000 patients that can use statistical techniques to identify prognostically relevant cTn thresholds.
Clinical Considerations
Numerous published studies with hs-cTn assays have now conclusively shown that cTn concentrations that are elevated, yet below the 99th percentile, define a higher-risk group of patients. This new generation of assays not only detects absolute values and dynamic changes in cTn concentrations, but also appears to expedite diagnosis of ACS (12, 14, 33) and help identify patients with pre-existing cardiovascular disease who are at increased risk of recurrent events (34).
Laboratorians will need to help clinicians understand how to effectively use the results of hs-cTn assays, especially because optimal thresholds for the percentage of absolute change have not yet been defined for the assays coming into use. Similarly, clinicians will need to recognize the tradeoff between sensitivity and specificity, and that all manufacturers’ assays are not equivalent.
With the 10-fold higher sensitivity of the new hs-cTn assays, clinicians will now find that most patients, even those without apparent disease, have detectable cTn levels (11, 12, 15). Although these elevations are likely to be cardiac in origin (33), it is unclear at present how clinicians should respond to them other than by evaluating serial values (19, 35, 36). Clearly, however, we do know that cTn elevations have prognostic value: the higher the values, the greater the risk even within the normal range of values (8, 37).
Laboratorians will also want to keep apprised of other emerging and important clinical uses of these assays, including in stable coronary artery disease (33, 37), stress testing scenarios (38), and heart failure (39).
Final Thoughts
Although the clinical implications of using hs-cTn assays are not completely clear at present, these assays undoubtedly hold promise for better understanding the basis of ischemic cardiac injury and predicting prognosis with ACS. We fully expect that these forthcoming assays will be able to reliably detect cTn concentrations at or below the 99th percentile cutoff, as well as document delta values more accurately as specified by the universal definition of MI.
SUGGESTED READING
Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–69.
Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61.
deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–502.
Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–61.
Kavsak PA, Allen LC, Apple FS, et al. Cardiac troponin testing in the acute care setting: ordering, reporting, and high sensitivity assays—an update from the Canadian society of clinical chemists (CSCC). Clin Biochem 2011;44:1273–7.
Kavsak PA, MacRae AR, Yerna MJ, et al. Analytic and clinical utility of a next-generation, highly sensitive cardiac troponin I assay for early detection of myocardial injury. Clin Chem 2009;55:573–7.
Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations. J Am Coll Cardiol 2012;59:1570; author reply 1–2.
Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684–93.
Morrow DA, Cannon CP, Rifai N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: Results from a randomized trial. JAMA 2001;286:2405–12.
Venge P, Johnston N, Lindahl B, et al. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol 2009;54:1165–72.
Wu AH, Apple FS, Gibler WB, et al. National Academy of Clinical Biochemistry Standards of Laboratory Practice: Recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem 1999;45:1104–21.
Wu AH, Jaffe AS. The clinical need for high-sensitivity cardiac troponin assays for acute coronary syndromes and the role for serial testing. Am Heart J 2008;155:208–14.
 Sabu Thomas, MD, FACC, FRCPC, is an assistant professor in the Division of Cardiology, University of Rochester School of Medicine, Rochester, N.Y.
Email: [email protected]
Sabu Thomas, MD, FACC, FRCPC, is an assistant professor in the Division of Cardiology, University of Rochester School of Medicine, Rochester, N.Y.
Email: [email protected]
Disclosure: The author has nothing to disclose.
 Peter Kavsak, PhD, FCACB, FACB, is an associate professor in the Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ont., Canada.
Peter Kavsak, PhD, FCACB, FACB, is an associate professor in the Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ont., Canada.
Email: [email protected]
Disclosure: The author has a financial interest in Abbott, Beckman, Randox, and Roche.

P.J. Devereaux, MD, PhD, FRCPC, is an associate professor in the Departments of Clinical Epidemiology and Biostatistics and Medicine, McMaster University, Hamilton, Ont.
Email: [email protected]
Disclosure: The author has a financial interest in Roche Diagnostics.