Walking around the AACC 2012 Clinical Lab Expo in July, attendees could not miss the many exhibits featuring large, multifaceted analyzers—the latest incarnations of central lab instruments that are the mainstay of hospitals and reference labs. Their gleaming insides of pumps, wires, tubes, and robotics suggest the power and precision required for instruments to turn around thousands of tests an hour.
Of course, diagnostic testing is no longer limited to these traditional big box machines. The large analyzers and automation lines shared the spotlight with innovative point-of-care (POC) devices on the exhibit floor, as companies have pushed the envelope to make POC faster, easier, and more reliable. This year, more than 120 companies came to Los Angeles to showcase POC products. Some of these new products brought refinements to well-established areas that the majority of hospitals now depend on, such as blood gases. Others aimed to take more testing out of the central lab and into physician offices.
The buzz about POC also was evident during educational sessions at the AACC Annual Meeting. Speakers covered many POC topics, including the explosion of POC technology and where POC opportunities lie. According to Matthew Thompson, MD, the biggest untapped opportunities remain in primary care. "In some ways, we have a perfect storm for diagnostic innovation in primary care—nearly a billion visits to ambulatory physicians in the U.S. each year, and misdiagnosis is the most common cause of malpractice lawsuits," he said. "However, currently there is a massive mismatch, with technological capabilities far outweighing adoption." Thompson, a senior scientist and director of the Centre for Monitoring and Diagnosis at the University of Oxford in the U.K., spoke at an AACC Annual Meeting symposium titled, Meeting the Needs of Patients and Primary Care Physicians.
Innovation First
The definition of POC instruments implies innovation in both form and function. After all, these devices must be easy enough to be used by healthcare providers outside the lab, and robust enough to survive the demands of everyday use. Accurate results are also essential.
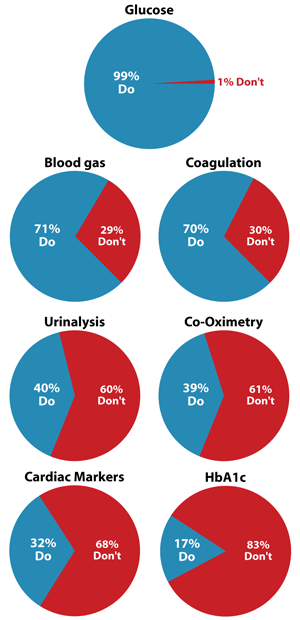
One of the areas where POC testing has carved out a place for itself is in the measurement of blood gases. According to a recent report from the strategic consulting company Enterprise Analysis Corporation, 71% of hospitals surveyed in 2011 now rely on POC blood gas instruments, a number surpassed only by POC blood glucose testing (See Box, below).
The Growth of Point-of-Care Testing in Hospitals
Cardiac Markers Fuel Growth in $5 Billion Market
Hospital point-of-care (POC) testing continues to grow steadily, with hospitals adding more instruments and rapidly increasing the volume of cardiac testing in particular, according to a new report from strategic consulting firm Enterprise Analysis Corporation (EAC). This brief analyzes data from a 2011 survey of hundreds of hospital-based point-of-care coordinators (POCC), and compares data to the company’s previous survey in 2007.
While POC glucose, blood gas, coagulation, and HbA1c tests have seen a slow, steady rise over the past 4 years, cardiac marker testing has risen sharply, according to EAC senior consultant Michelle Keane. “We were surprised to find 10 percent more hospitals performing cardiac marker testing compared to 2007, and volumes have increased significantly as well,” Keane commented. For stand-alone troponin, nearly twice as many hospitals surveyed by EAC now perform POC testing.
Two instruments continue to dominate the cardiac marker POC market, Abbott’s i-STAT system and Alere’s Triage system. The Triage has seen a 70% increase in volume for cardiac tests, and i-STAT’s volumes have tripled.
Keane also noted that POC has become more complex in hospitals, with 23% of institutions reporting use of six or more different POC instruments in 2011 compared to just 5% in 2007. Nearly 50% of hospitals EAC surveyed now have four or more instruments.
Hospitals Performing POC Testing
Roche Diagnostics came to the AACC Clinical Lab Expo this year with a new POC blood gas system that, like many other new POC instruments, aims to meet a triple goal: reliable operation, economy, and accuracy. The new cobas b 123 system deals with a common problem that plagues such instruments—stubborn blood clots that interfere with the system's delicate sensors. Unlike blood specimens for chemistry tests that clot and are centrifuged before testing, blood gas testing requires samples to remain in a full, fluid state for analysis. But the heparin in blood tubes cannot always prevent clotting, leading to reagent pack failures in blood gas instruments, explained Mary Catherin Coyle, MT(ASCP), director of product marketing at Roche Diagnostics. "Hospitals needed a machine that would prevent the clot from entering the instrument, or if it would enter, send it directly to the waste. And that's exactly what we were able to do with the b 123."
To solve the blood clot problem, the b 123 features several layers of defense. It uses an adapter that catches blood clots right before the entry port out of the syringe, and further into the system, several pinch points block clots to protect internal sensors. When a clot reaches a pinch point and disrupts the instrument's ability to pull the sample through, the clot is shot out into a waste container.
Roche's blood clot solution exemplifies the kind of refinements in mechanical engineering that now drive some segments of the POC testing market. According to Coyle, blood gas testing volumes are expected to remain stable. "These systems are holding their own, but will likely not see a large expansion," she said. To stay competitive, companies will increasingly add assays to blood gas analyzers, Coyle added. For example, the b 123 can analyze oxygen, carbon dioxide, pH, hematocrit, sodium, potassium, calcium, glucose, lactate, total hemoglobin, sulfur dioxide, oxyhemoglobin, deoxyhemoglobin, carboxyhemoglobin, and methemoglobin.
With an eye on the economical use of reagents, Roche also designed the b 123 with special read/write electronic memory chips embedded in reagent packs. These smart chips carry the expiration of the lot number, as well as each reagent's on-board life. The instrument will not allow reagent use beyond these expiration dates. The chips on reagents, quality control packs, and linearity packs allow these to be interchangeable among instruments, which can then capture the lot and on-board life data. "This allows our customers to take full advantage of reagents down to the last drop," Coyle said. "For example, in a case where a surgery suite is only open Monday through Friday, the staff could on Friday take that pack off that still has reagents or sensor life and move it to another instrument in the emergency department."
The new Roche instrument also illustrates a trend towards miniaturization for POC instruments. Miniaturization is all about delivering lab-quality results on a smaller scale, rather than going back to the drawing board to work out an entirely new method, Coyle explained. "Our approach was to use all of the same technologies as a large analyzer in the lab," she said. "The same kind of electrodes and test tubes are there, just very small, and we took the effort to put in the highest class carbon monoxide-oximeter available."
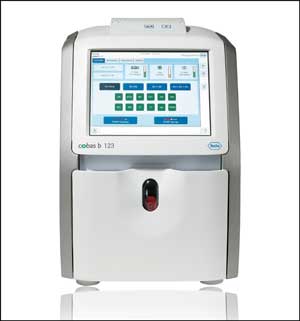 The new Roche cobas b 123 instrument features unique engineering
to avoid blood
The new Roche cobas b 123 instrument features unique engineering
to avoid blood
clots which often contribute to down-time in blood gas instruments.
Moving Farther From the Lab
While POC devices have moved certain types of tests out of the lab and into various areas of the hospital, many manufacturers are looking to make further inroads into physician offices, clinics, and even pharmacies. One early-stage company at this year's AACC Clinical Lab Expo, Ireland's Biosensia, exhibited its compact RapiPlex system for the first time. The desktop RapiPlex platform uses novel technology that can run within 15 minutes up to 12 immunoassays from a tiny whole blood or saliva sample.
From its inception, Biosensia design-ed the system for CLIA-waived use in settings far from the lab, according to CEO Diarmuid Flavin. A CLIA waiver from the Food and Drug Administration (FDA) allows non-laboratory personnel to perform a test in a wide variety of settings, thereby creating more opportunities for manufacturers to sell their devices.
"The move towards more decentralized testing will drive demand for this type of technology," he said. "We've seen evidence from market reports that the number of tests performed at the point-of-care as a function of the total number of diagnostic tests performed annually will increase five percent by 2018 globally. And our RapiPlex system, which enables semi-skilled users to perform tests in an environment like a pharmacy or a doctor's office, can open up a whole new segment for multiplex panels."
The RapiPlex system consists of single-use immunoassay test cartridges and a fluorescence reader with a touch-screen interface. The test cartridge houses the core engineering in this platform. Unlike lateral flow tests that laboratorians are familiar with, the RapiPlex employs a vertical flow microfluidic design that does not rely on capillary flow between materials. The cartridges depend on simple gravity to pull the sample first through a red blood cell filtering membrane, then a fluorescent antibody conjugate pad, and finally a nitrocellulose membrane printed with antibody capture lines.
Such a self-contained, microfluidic system can deliver on accuracy, ease-of-use, and cost efficiency, according to Flavin. "Because it is a passive system, it allows simple steps for the user without worrying about expensive mechanical washing, pumps, or other elements," he said. "This also translates into a competitive advantage in terms of cost for both the reader and the cartridges."
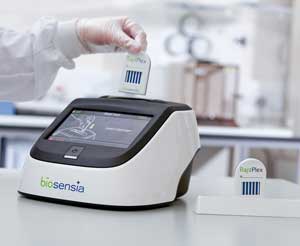 The Biosensia RapiPlex platform uses innovative, vertical-flow
microfluidics to analyze up to 12 analytes at a time.
The Biosensia RapiPlex platform uses innovative, vertical-flow
microfluidics to analyze up to 12 analytes at a time.
As a young company, Biosensia came to the Clinical Lab Expo looking for partnerships to commercialize assays. In the meantime, the company plans to seek FDA clearance for a cardiac product, as well as a CLIA-waived dual syphilis test that combines the screening and confirmatory tests in a single application. These first FDA-cleared assays will demonstrate that the platform is ready for clinical use and give potential business partners confidence in the technology, Flavin said.
Flavin sees a lot of opportunity for RapiPlex, especially for use by physician group practices. "A single doctor's office is becoming a thing of the past now, when we have groups of physicians opening practices together," he said. "These groups often seek to add a higher degree of service to their patients within the same office, with more offerings such as physiotherapy or diagnostic testing. It's about giving more value to patients."
To reach as many physician practices as possible, reimbursement has some catching up to do, Flavin emphasized. "One of the challenges that industry faces here is the ability to reimburse multiplex products—something that technologies like ours are trying to influence," he said. "It could be a pretty difficult up-hill climb without appropriate reimbursement. However, we know the opportunity exists here."
Opening Doors in Primary Care
Data show that despite its premium price, hospitals are still eager to adopt POC testing, especially in areas like the emergency department (See Box, p. 6). However, beyond urine dipsticks and other simple tests, POC instruments have yet to stake a claim in many primary care settings.
In some ways, primary care physicians still rely most on tools that have changed little in 100 years, noted Thompson at the AACC Annual Meeting symposium. "I'm a family doctor, and in my practice, when the patient comes into the room, they'll see a desk, a chair, and computer. For tools, I have little more than a light to look in their ears and a stethoscope," he said. "So when you look around the AACC Clinical Lab Expo and see the hundreds of exhibits with all these tests, some of which can be done with just a drop of blood, I feel frustrated that physicians don't quite seem to be practicing in the 2012 world of technology I see on the exhibit floor."
Thompson and a group of researchers at the U.K.'s Centre for Monitoring and Diagnosis (MaDOx) are working to close this technology gap by looking at the needs of primary care physicians and systematically evaluating POC technologies. The MaDOx research program is funded by the U.K. National Institute for Health Research (NIHR) based in the Department of Primary Care Health Sciences at the University of Oxford. MaDOx works to summarize available research on high-priority POC tests and refine new research questions that remain unsolved (More available online). These Horizon Scanning Reports, as they're called, are then used by NIHR to guide funding that can demonstrate which POC tests should be pulled in to primary care offices. Horizon Scanning Reports include a systematic search of extant litera-ture and employ critical appraisal tools developed by the University of Oxford's Centre for Evidence Based Medicine (More available online).
"I think a lot of this slowness to adopt point-of-care technologies has been due to a lack of the right research studies that ask the right kinds of questions," Thompson said. "We need more research studies that prove these tests actually improve care and are cost-effective." Many studies of POC tests focus almost exclusively on technical accuracy, he added. Instead, MaDOx is pushing for research that tackles more holistically reliability, diagnostic accuracy, impact on outcomes, and cost-effectiveness.
For example, a MaDOx Horizon Scanning Report published in March examined procalcitonin (PCT) POC testing for improving the early diagnosis of serious bacterial infections in patients presenting in primary care. The report summarizes the current literature on the subject and distills six unsolved research questions: What is the accuracy and precision of POC PCT tests in the primary care setting? What is the usefulness of POC PCT tests in targeting antibiotic prescriptions in patients presenting with acute infections to primary care? In primary care patients presenting with acute infection, is a POC PCT test able to exclude serious bacterial infections? Does a POC PCT test combined with a clinical prediction rule aid diagnoses of acute infections? What is the ideal healthcare setting where POC PCT tests would have the greatest impact on confirming or excluding serious bacterial infections? What is the cost-effectiveness of POC PCT testing in primary care?
Part of Thompson's research also focuses on understanding how primary care physicians use diagnostics in the first place, and what kinds of technology they feel would help them. He emphasized that a large part of primary care encompasses assessing patients with vague and unexplained symptoms. "Many patients present with symptoms like headaches, fatigue, or abdominal pain, and in most of these cases, the complaint doesn't turn out to be anything serious," he said. "Not everyone coming in with a bit of abdominal pain needs anything more than an examination and their history taken—they don't all need CT scans, ultrasounds, blood tests, and a colonoscopy. So trying to decide who to do tests on and who not to do tests on is part of the art of medicine in primary care."
As a result, physicians do not really want or need every single blood test right away, Thompson explained. "Point-of-care tests are only going to be useful when they provide an answer that will direct your patient management at that very moment," he said. "But, for example, if someone is tired and you check this individual's thyroid level, whether one has the answer today or next week is not critical." Monitoring patients with chronic diseases such as diabetes or heart disease is also an area of opportunity, Thompson added, since some medications can be adjusted on the spot.
In 2011, Thompson and several of his MaDOx colleagues convened a meeting of physicians, researchers, and diagnostics manufacturers that addressed the technology-adoption gap. In their report of the meeting's outcome, "Innovation in Diagnostics and Healthcare: Improving Bench to Bedside Processes for Testing," the top recommendation was to encourage early engagement among clinicians and industry.
As more care shifts to primary care, the pressure will only increase on physicians and manufacturers—as well as on government and private payers—to decide how POC tests will be reimbursed and used outside of hospitals. "Care is moving out of hospitals into primary care clinics and even pharmacies," Thompson said. "And patients have growing access to rapid testing over-the-counter or on the Internet, such as the rapid HIV test approved for sale this year by FDA. Patients are growing to expect rapid answers, and they will not want to wait even 24 hours for a test result."
Q&A with AACC Point-of-Care
Coordinator of the Year Pet Maniquis

A medical technologist with 40 years' experience in the field, Pet Maniquis, MPA, MT(ASCP), won the 2012 AACC Point-of-Care Coordinator (POCC) of the Year award, presented during a Point-of-Care Coordinators Forum at the AACC Annual Meeting. Maniquis has many years of experience in the lab, including working as special chemistry supervisor, forensic analyst, and laboratory manager at Bethany Medical Center in Kansas City, Kan., and training in forensic analysis at the Federal Bureau of Investigation lab in Arlington, Va. Maniquis also served as an expert toxicology witness until 2000.
Since 2001, Pet has worked as the POCC for Providence Medical Center and Saint John Hospital, both in Kansas City. She now manages POC testing (POCT) for 18 hospitals, surgical centers, rehab hospitals, and physician offices in Kansas and Missouri.
How did you come to manage so many sites?
Physicians Reference Lab, a local private lab, took over the management of the laboratory here at Providence Medical Center in 2003. The reference lab also had other hospital clients with POCT needs. PRL did not have a POCC before, so when they asked for help, I began managing these additional sites. I started with one or two, and then after that, they included POCT at every hospital they signed a contract with. So I would go in there, set them up, and do quality monitoring. Now I have eight hospitals total, as well as 10 clinics that Providence Medical Center has opened.
Do you see the responsibilities of POCCs expanding?
Absolutely, and it’s a trend we can’t stop. Many of the medical technologists are getting older—we have to retire sooner or later—and there are not that many young people going into the clinical lab field. Most of the medical technology students that I know who train at Kansas University Hospital in Kansas City want to specialize in molecular testing. So, there will be fewer of us with the clinical lab experience who may be interested in the job.
How are you able to stay on top of so many sites?
For me, organization is key. I have to visit each site at least once each month to make sure the quality control is acceptable and talk with the risk management director or nurse manager. Every six months, I perform calibration verification of each non-waived instrument at each site. This takes a lot of time because there are a lot of instruments. Some hospitals have as many as 14, others just one or two. Each site has its own computer system, so I cannot monitor anything remotely. I have to be out in my car even in rain or snow visiting all of these sites.
I make sure each site complies with the most stringent accreditation standards. I work with four different accreditation organizations and each one is a little bit different in their requirement. But my philosophy is to make everyone follow the same requirement to meet the highest standard.
What keeps you motivated?
The aspect of my job that I like the most is being able to teach about lab work. I like explaining to the POC users how and why POC tests are important even though they appear very simple. For example, it is important that users follow proper procedures for collecting samples. I often need to explain why collecting the sample in the correct color tube is important, and that not all anticoagulant tubes are created equal.
Meeting and interacting with nursing personnel also keeps me interested in my job. I get to see their point of view when I am around them. Nothing is routine when I am outside of the lab. There is always a challenge. And when my POC sites pass their inspections, often the CEOs and the nursing directors personally thank me, which really makes me feel good.