The androgen steroid hormone, testosterone, plays a significant physiological role in both men and women, so being able to measure it accurately and reliably has important clinical implications. In men, testosterone test results are crucial in diagnosing hypogonadism, and in women, they assist in the work-up of suspected polycystic ovary syndrome. Testosterone measurements also aid in monitoring treatment response in men taking enzyme inhibitors for prostate cancer.
However, a number of concerns exist about the reliability of testosterone assays, especially when it comes to measuring free testosterone with homogenous assays. Other issues involve the ability of assays to detect subtle differences in testosterone levels, for example identifying individuals with androgen deficiency or detecting low levels in women and children. Additionally, comparability of testosterone data from different assays is lacking, which makes it very difficult for researchers to compare and compile data and to develop evidence-based guidelines.
Given these challenges, the Centers for Disease Control and Prevention (CDC), with broad input from various professional societies, is leading a Hormone Standardization Program with an initial focus on standardizing testosterone measurements. Standardization in measuring any analyte is desirable so that test results are accurate and reliable independent of both the assay used and the time and place of measurement. In this article, we will outline the scope, progress of, and next steps for this important initiative.
Testosterone Standardization Program
In collaboration with The Endocrine Society, CDC held a workshop in 2008 to discuss concerns and challenges in testing testosterone and other steroids. Participants representing several professional societies such as the American Association for Clinical Chemistry, The Endocrine Society, and the American Cancer Society, addressed clinical and research applications of steroids in areas as diverse as male androgen deficiency, cancer, pediatrics, and reproductive medicine. Based on recommendations from the participants, CDC started the Hormone Standardization Program with an initial focus on standardizing testosterone measurements.
The conceptual approach of the testosterone standardization program is built on experiences gained from successful standardization programs CDC maintains or has supported, such as the cholesterol standardization program and the National Glycohemoglobin Standardization Program. The testosterone standardization program consists of three basic steps: developing a reference system, calibrating individual assays, and verifying end-user test performance (Figure 1).
Figure 1
Steps for Achieving Accurate and Comparable Patient Results
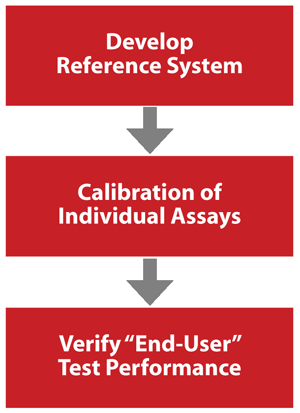 The first step is to establish a point of reference by developing a reference method and materials. In the second step, these reference methods and materials are used to calibrate assays and assure that they have an appropriate accuracy and precision. The last step involves verification of end-user performance to ensure actual patient results are accurate and comparable.
The first step is to establish a point of reference by developing a reference method and materials. In the second step, these reference methods and materials are used to calibrate assays and assure that they have an appropriate accuracy and precision. The last step involves verification of end-user performance to ensure actual patient results are accurate and comparable.
Creating a Reference System
As the first step towards creating a reference system for testosterone, CDC developed a reference method using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC/MS/MS) that was calibrated with the pure compound reference material, NMI-M914, from the Australian National Metrology Institute. SRM 971, a serum-based reference material from the National Institute for Standards and Technology (NIST) served as the trueness control. CDC also initiated further comparison studies with other reference laboratories, including those of Linda Thienpont, PhD, at the University of Ghent (Belgium) and Susan Tai, PhD, at NIST, to assure accuracy and comparability to other reference laboratories. Through these activities, the CDC reference laboratory assured metrological traceability as described in ISO 17511.
Using its testosterone reference method, CDC is in the process of assigning values to single-donor sera, which are produced following a standard protocol developed by the Clinical Laboratory and Standards Institute (Protocol C37-A). Sera obtained with this protocol can be considered commutable, meaning they contain minimal matrix effects and behave very similar to regular patient samples. Use of commutable sera enables immunoassay manufacturers, as well as laboratories operating laboratory-developed MS–based tests, to use the materials as calibrators or trueness controls. This ultimately assures that measurements in patient samples are accurate and comparable.
Calibrating Individual Assays
Assay calibration also is critical to measuring testosterone accurately and reliably. CDC is therefore working with immunoassay manufacturers to help calibrate their assays, as well as with laboratories on their lab-developed tests. These activities are being accomplished through the CDC Hormone Standardization Program (HoSt) started in 2010. The HoSt Program is open to all manufacturers and laboratories interested in accurate testosterone testing.
The HoSt Program consists of two phases (Figure 2). The first involves assessing participants' accuracy and performance in measuring testosterone and subsequently addressing any performance problems. The second phase involves evaluating HoSt Program participants' measurement performance and calibration stability via quarterly blinded challenges conducted during a 12-month period. The data from all four challenges are used to determine measurement bias, which is compared to the desirable measurement bias of 6.4%. Laboratories that meet this bias criterion are considered standardized to CDC.
Figure 2
CDC Hormone Standardization (HoSt) Program Process
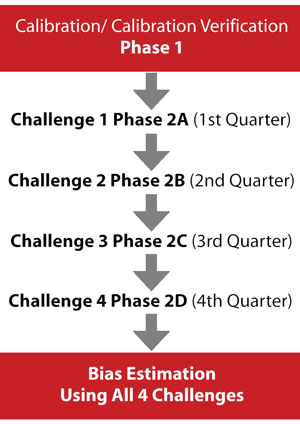 In Phase 1, single donor patient samples with known values are provided to the participating laboratory to assess and adjust calibration. In Phase 2, the laboratory is challenged four times with 10 samples each quarter. After the fourth challenge, data from all challenges are used to assess bias to the CDC reference method. Laboratories with a mean bias ± 6.4% are considered standardized to CDC and sufficiently accurate.
In Phase 1, single donor patient samples with known values are provided to the participating laboratory to assess and adjust calibration. In Phase 2, the laboratory is challenged four times with 10 samples each quarter. After the fourth challenge, data from all challenges are used to assess bias to the CDC reference method. Laboratories with a mean bias ± 6.4% are considered standardized to CDC and sufficiently accurate.
HoSt Program performance criteria are derived from data on the biological variability of testosterone. The concept of using biological variability to derive measurement performance criteria was suggested during a 1999 conference in Stockholm on strategies to set global analytical quality specifications in laboratory medicine. Using biological variation to drive analytical performance sets the minimum criteria of analytical variability to detect true biological or physiological changes.
Now in its second year, the HoSt Program for testosterone has 20 participants, all at varying stages of the program. Eight have successfully completed at least 1 year of Phase 2 (Figure 3). Information about participants meeting current performance criteria is available online. The information is updated quarterly and certification is valid for 1 year.
Figure 3
The CDC HoSt Program
These laboratories have successfully performed the CDC standardization program. Certification is valid for one year.
- Endocrine and Metabolic Research Lab of Los Angeles Biomedical Research Institute, LC/MS/MS
- LabCorp, LC/MS/MS
- Boston University Steroid Hormone Assay Laboratory, LC/MS/MS
- Roche Diagnostics GmbH, Electrochemiluminescence
- Quest Diagnostics, LC/MS/MS
- Mayo Clinic, LC/MS/MS
- ARUP Laboratories, Inc., LC/MS/MS
- Covance Central Laboratories Services, LC/MS/MS
Updated May 2012. Go to the CDC website.
CDC also provides Phase 1 material to those laboratories not able to participate in the certification portion of the HoSt Program. Furthermore, to assure the accuracy of testosterone testing over time, especially as reagents and methodologies change, the program offers ongoing assistance with calibration and performance evaluation to the clinical laboratory community.
Verifying End-User Test Performance
In addition to establishing a reference system and helping calibrate individual assays, CDC collaborates with proficiency testing (PT) programs to assess the accuracy of testing performed in clinical laboratories. Using its testosterone reference method, CDC assigns reference values both for the College of American Pathologists (CAP) accuracy-based survey for testosterone and estradiol and to materials used in the New York State PT program. The CDC testosterone standardization program also provides accuracy-based quality control materials to investigators and laboratories performing large epidemiological studies and clinical trials, thereby enabling them to monitor the accuracy of measurements in these studies over time.
Data from the New York State PT program show the initial impact of the CDC standardization program (Figure 4). Variability expressed as the coefficient of variability in data among mass spectrometry laboratories decreased from 2006 to 2011, indicating that improvement in the accuracy of testosterone measurements is being achieved.
Figure 4
Testosterone Interlaboratory Variability
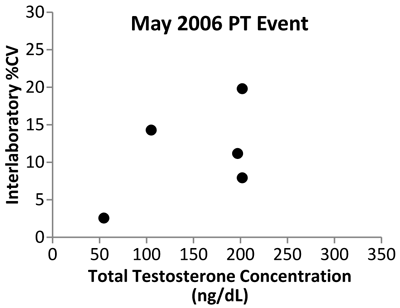
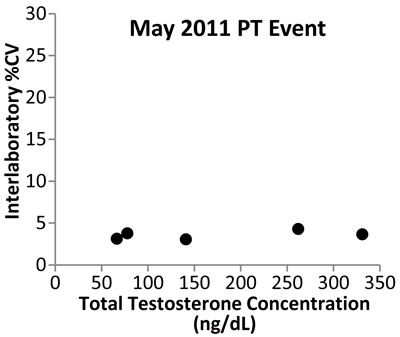 Shown are data from the New York State Proficiency Testing program expressed as percent of coefficient of variation (%CV). The study used five samples analyzed by MS-based assays in May 2006, before the start of the CDC standardization program, and in May 2011, after 1 year of standardization. The variability between laboratories decreased from 2006 to 2011, with all participating laboratories reporting values within 5% of each other for all five test samples.
Shown are data from the New York State Proficiency Testing program expressed as percent of coefficient of variation (%CV). The study used five samples analyzed by MS-based assays in May 2006, before the start of the CDC standardization program, and in May 2011, after 1 year of standardization. The variability between laboratories decreased from 2006 to 2011, with all participating laboratories reporting values within 5% of each other for all five test samples.
The Benefits of Testosterone Standardization
Accurate and reliable testosterone measurements are beneficial in many ways. For example, they support establishment of clinical guidelines, such as The Endocrine Society’s guideline that recommends specific testosterone levels as an aid to diagnosing androgen deficiency in men. Standardization also facilitates the use of common testosterone reference ranges, which not only saves laboratories time but also the resources required to establish their own reference ranges. Together these benefits allow further assessments of research findings across studies, which in turn facilitates translation of research findings into clinical practice.
One illustration of how standardization impacts lab practices and patient care comes from a collaboration CDC formed with Shalender Bhasin, MD, professor of medicine and section chief of endocrinology, diabetes and nutrition at Boston University. Dr. Bhasin conducted a study to establish normal testosterone ranges for males using the Framingham Gen 3 study population. He standardized his laboratory measurements to the CDC methods, and CDC provided accuracy-based quality control materials to assure accuracy of study data over time. As a result, it now will be easier for other CDC-standardized laboratories to use the normal ranges established by Dr. Bhasin in this study.
In addition, CDC currently is using its isotope dilution HPLC/MS/MS assay to analyze samples from men, women, and children age 6 years and older in the 2011/2012 NHANES cycle. Because assays are standardized, data from NHANES can be compared easily with data from the Framingham study. Furthermore, CDC-standardized labs can compare their data to the NHANES results.
Partnership for Accurate Testing of Hormones
In 2010, The Endocrine Society convened a consensus conference with representatives from the National Institutes of Health, Food and Drug Administration, CDC, and other professional organizations and stakeholders, with the goal of creating a framework to implement standardized testosterone measurement. This conference led to both a consensus document endorsed by 11 organizations and formation of the Partnership for the Accurate Testing of Hormones (PATH). PATH serves several purposes, not the least of which includes providing ongoing advice and coordinating activities that facilitate the use of standardized hormone tests. PATH also monitors the progress of standardization efforts and promotes the use of standardized testing through education and policymaking.
PATH’s Technical Advisory subcommittee, chaired by AACC representative, Robert Fitzgerald, PhD, provides scientific and technical expertise to support standardization. The subcommittee is currently reviewing research data on biological variability to re-evaluate current performance criteria. The findings from this study will help to further refine method performance criteria and to identify data gaps. PATH also is working with Dr. Bhasin to establish normal ranges in men.
Towards Better Patient Care
Standardization of clinical laboratory measurements improves the diagnosis and treatment of diseases. Achieving standardization requires collaboration among all groups and stakeholders involved in the measurement process and use of the measurement results. The CDC testosterone standardization program is an example of how different organizations and stakeholders can work together successfully to improve patient care and public health through better diagnosis and treatment of diseases.
CDC is conducting similar collaborative initiatives to standardize other biomarkers for cardiovascular disease, cancers, and bone health. For example, vitamin D is now part of the Vitamin D Standardization Program (VDSP), a collaboration with the Office of Dietary Supplements at the National Institutes for Health, and estradiol has been added to the HoSt Program this year. For further information on the CDC Hormone Standardization Program contact [email protected] or visit the CDC website.
SUGGESTED READINGS
Clinical Laboratory Standards Institute. Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures (CLSI document C37-A). Wayne, Pa: Clinical Laboratory Standards Institute; 1999.
Herold DA, Fitzgerald RL. Immunoassays for testosterone in women: better than a guess? Clin Chem 2003; 49:1250–1251.
Matsumoto AM, Bremner WJ. Serum testosterone assays—accuracy matters, J Clin Endocrinol Metab 2004;89:520–524.
Rosner W, Vesper HW, Endocrine Society and endorsing organizations. Toward Excellence in Testosterone Testing: A Consensus Statement. J Clin Endocrinol Metab 2010; 95:4542–48.
Rosner W, Vesper HW. CDC Workshop Report: Improving steroid hormone measurements in patient care and research translation. Steroids 2008;73:1285.
Sacks SS. Are routine testosterone assays good enough? Clin Biochem Rev 2005; 26:43–45.
Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev 2007;16:1713–1719.
Swerdloff RS, Wang C. Free testosterone measurements by analog displacement direct assay: old concerns and new evidence, Clin Chem 2008;54:458–459.
Vesper HW, Botelho JC. Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol 2010;121: 513–519.

Hubert W. Vesper, PhD is the director of Clinical Standardization Programs and Chief of the Protein Biomarker Laboratory in the Clinical Chemistry Branch, Division of Laboratory Sciences at the CDC, Atlanta, Ga.
Email

Julianne Cook Botelho, PhD is the lead research chemist in the hormone reference Laboratory and Standardization Program in the Clinical Chemistry Branch, Division of Laboratory Sciences at the CDC, Atlanta, Ga.
Email
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.