
SDI Productions / iStock
Whole-genome sequencing (WGS) will almost certainly replace gene panels in identifying cancer, calling for new healthcare infrastructures to support this trend in personalized care, argue U.K.-based cancer researchers Jyoti Nangalia, MB BCh, PhD and Peter Campbell, PhD in a New England Journal of Medicine review. Current debates about testing mutations with gene panels versus WGS are “transient and distracting,” according to the authors. “Ultimately, there is little doubt that we will be sequencing whole genomes.”
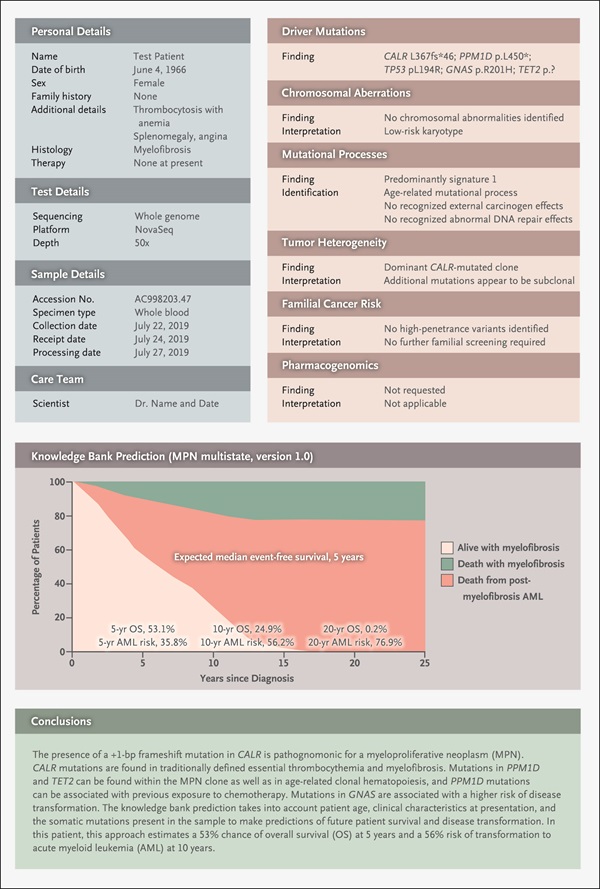
Given this inevitability, Nangalia and Campbell have put forth their vision of a future cancer genomics diagnostics report based on the profile of one of their patients with a myeloproliferative neoplasm:
Reproduced with permission of New England Journal of Medicine
Nangalia and Campbell envision an overall summary page with a high-level interpretative comment, linking out to supporting data and evidence. The mock report has 12 sections, including four dealing with details about the patient, test, sample, and care team. Six sections elaborate on the tumor’s genomic analysis, such as its driver mutations and chromosomal aberrations. The report also features a knowledge bank prediction that shows graphically the patient’s odds of survival at 5, 10, and 20 years based on the tumor’s genomics and the patient’s age and clinical characteristics at presentation.
Making the best use of this type of diagnostic report would require supporting elements such as:
- A new generation of diagnostic lab scientists who know the ins and outs of genomic sequencing, with quick access to genomic databases;
- Comprehensive, national quality assurance and improvement programs to harmonize the sequencing, analysis, and interpretation of cancer genomes;
- Phase 4 clinical trials that develop methods for amassing comprehensive data on genomics and clinical outcomes from patients who receive drugs after licensing;
- National and international initiatives for creating knowledge banks of patient data and data storage and access innovations; and
- Algorithms that support physicians in making care decisions for patients; and
- An ethical framework that promotes transparency in sharing data while protecting patient identity and privacy wishes.
All of these goals are within reach and in some cases, already taking place on a regional or national scale, the authors note. State-of-the art pilot programs “have begun the process of transitioning cancer genomics from academia to a sustainable, routine, and, with time, universally accessible diagnostic test underpinning cancer care,” they wrote.