 Image credit: Presage Biosciences
Image credit: Presage Biosciences
Research teams working separately at Massachusetts Institute of Technology (MIT) and the Fred Hutchinson Cancer Research Center reported developing tiny implantable devices that one day could be used to test an individual cancer patient’s response to chemotherapy before their tumors have been removed. The devices could be used to guide therapy for individual patients, and they might speed up drug development by testing the efficacy of new chemotherapeutic agents. The findings of both teams were published simultaneously in Science Translational Medicine.
The implantable microdevice developed at MIT—just 3 millimeters long—delivers up to 16 different drugs from micro-pockets within the device. The investigators tested it in mouse models of melanoma, prostate, and breast cancer. It is implanted in a tumor with a biopsy needle, then drugs are released into the tumor. After 24 hours, the device is removed with a coring needle and the tumor tissue analyzed to see which drugs produce the best response. The authors compared device results with systemic administration of the same drugs, and found the device-delivered micro results were comparable to the systemic response.
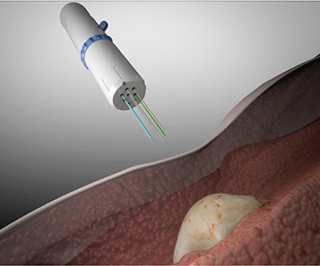
The device developed at Fred Hutchinson Cancer Research Center—dubbed CIVO—is a hand-held microinjection platform designed for tumors near the skin surface, like lymphoma, skin, or some breast cancers. CIVO has six needles, enabling drugs to be injected directly into the tumor and leaving a 6-mm track of the drug and an inert tracking dye. Researchers tested it in mouse and dog models, as well as four lymphoma patients. The lymphoma patients reported only mild discomfort and no serious side effects from CIVO. Researchers found clear cell death in the areas surrounding injected microdoses of vincristine.
Both teams developed their devices to address both the challenge of choosing the best drug for individual cancer patients and the need to find a wider variety of effective chemotherapeutic agents. "Currently, only 7 percent of new oncology drug candidates that demonstrated anti-cancer activity in preclinical studies subsequently demonstrate sufficient efficacy in clinical trials to warrant [Food and Drug Administration] approvals," explained researcher Jim Olson, MD, PhD, a member of the Clinical Research Division at Fred Hutchinson Cancer Center, in a prepared statement. "As a practicing pediatric oncologist, I deal every day with the limitations of current cancer therapies, and I've made it my life's work to help find solutions to this challenge."
“These techniques offer a possible alternative to the “hit and miss” way of using anticancer drugs in patients that has unfortunately become accepted practice; instead, these devices offer a personalized system for assessing drug sensitivity in vivo and tailoring therapy accordingly,” added R. Charles Coombes in an accompanying editorial.
Both teams are working to further develop the devices.