For years, researchers have used mass spectrometry imaging to characterize tissue samples, determining cancerous versus benign material, for example, or identifying subtypes of inflammatory bowel disease. But with an increasing need for speed and accuracy to guide clinical decision-making, the technology has the potential to soon make its way into clinical laboratories.
In simple terms, mass spectrometry measures the weight of chemical species, explained Abraham Badu-Tawiah, PhD, a professor of chemistry at Ohio State University whose research focuses on the development of new mass spectrometry techniques for disease detection. Every chemical species has a specific mass determined by the elements in that compound, he said.
“If I can measure the mass, then I can figure out what elements are in that compound,” Badu-Tawiah said. The technology “gives you the information you need in a very short amount of time. It’s also very sensitive, so you don’t need a whole lot of sample, and it’s accurate.”
Mass spectrometry imaging (MSI) carries this precision one step further and allows researchers to visualize the distribution of molecules in a particular tissue sample, otherwise known as spatial omics research, said Yusheng Zhu, PhD, DABCC, FADLM, a professor of pathology and laboratory medicine at Penn State University. MSI “has high potential to be used clinically,” Zhu said. That’s because it can be used for multiplex analysis, e.g., measuring all given molecules in the same tissue or cell at the same time. This can include detecting proteins (proteomics), lipids (lipidomics) and glycans, or sugars (glycomics), etc., in situ, to help render a more precise diagnosis. It also has an advantage over other detection techniques that need sample extraction, he said, because during the typical extraction and preparation of samples, some information can be lost, precluding scientists from understanding the localization of molecules. In MSI, though, minimal is necessary.
MSI is similar to molecular pathology, but instead of probing just one molecule, the technology enables the study of thousands of molecules in one experiment, said Ron M.A. Heeren, PhD, director of the Maastricht MultiModal Molecular Imaging Institute, at Maastricht University in the Netherlands.
Today, MSI most typically is used in the research setting. But MSI holds particular importance in areas such as intraoperative diagnostics, Heeren said. When a cancer patient is on the operating table and the surgeon removes a portion of potentially cancerous tissue, that tissue needs to be evaluated during the procedure. Typically, the surgeon can wait 30 minutes or so to make a clinical decision, but that timeclock is tight for a pathologist, who may have to prepare samples with antibody or fluorescent stains, incubate cells, or retrieve antigens—all of which require time and several steps.
“Because mass spec imaging can image thousands of molecules at the same time, the acquisition of an informative mass spec image takes about 10 minutes,” he said. Even allowing for sample prep and data analysis, a decision can be rendered in under 30 minutes. “Mass spec imaging offers the potential to enhance clinical diagnosis in the timeframe of the surgical decision-making process, which means that it becomes much easier to determine surgical margins immediately during surgery.”
Some MSI tools are beginning to make their way to the operating rooms, Badu-Tawiah said. Take the intelligent knife (iKnife), referenced in one of his recent papers (Anal Chem 2020;92:183-292). The tool allows surgeons to test tissue upon contact during cancer removal operations, indicating to the doctor whether a particular area contains cancer cells. MasSpec Pen technology is also being trialed in cancer surgeries, to accurately identify cancer versus normal tissues and surgical margins upon contact. (Fun fact: The technology was featured in an episode of the TV show “Grey’s Anatomy”).
Livia Schiavinato Eberlin, PhD, who invented the MassSpec Pen, demonstrated the progress her research team is making with the technology in a plenary session at the 2022 AACC Annual Scientific Meeting in July. The device delivers a controlled, discrete water droplet to a tissue’s surface to extract biomolecules, which it then transports to a mass spectrometer for analysis. Her team is also exploring MSI.
MSI has huge potential, said anatomic pathologist Kristina Schwamborn, MD, PhD, a consultant at the Institute of Pathology, Technical University of Munich, in Germany.
The problem we’re facing in pathology nowadays is that there are regions in the body that can be reached to get biopsy material, but the material you get is very limited, and sometimes compromised by crush artifacts (damage caused by incorrect use of forceps handling the tissue). "Still, we have to come to a diagnosis,” Schwamborn said. This means first determining if a sample is cancerous or benign, for example. If it’s cancerous, then determining what type of cancer, and what mutations are present. “We have to answer a lot of questions.”
MSI allows the interrogation of multiple markers without rendering damage, so the same material could later be studied via traditional hematoxylin and eosin histology or immunohistochemistry staining, or additional molecular analysis, she said. It can compete with the gold standard pathology tests while being faster and cheaper.
“The worst thing is if you tell the clinician, ‘The sample that you gave me is not good enough. We need more.’ Or, ‘we can’t give you a definite diagnosis,’ ” Schwamborn said. “Any tool that can aid in that and tailor the treatment, defining whatever the patient has more carefully and more thoroughly, will aid ultimately in patient care, meaning going toward personalized medicine.”
Karen Blum is a freelance medical and science writer who lives in Owings Mills, Maryland. +Email: [email protected].
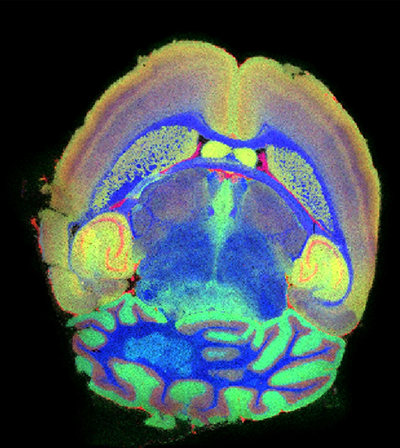
The image at the top of this article is a mass spectrometry image of healthy coronal mouse brain tissue section using TransMIT’s AP-SMALDI5 AF ion source coupled to the Thermo ScientificTM OrbitrapTM Exploris mass spectrometer. Mass spectrometric and position information are combined in a post-processing step using TransMIT’s MIRION software.
It shows three masses (actually ions of individual phospholipid species) extracted as three colors in a red-green-blue overlay ion image with red: m/z 820.5253, PC(36:4), [M+K]+, green: m/z 769.5620, SM(36:1, O2), [M+K]+ , and blue: m/z 826.5722, PC(36:1), [M+K]+.
The spatially localized lipids demonstrate various well-defined anatomical regions, such as the white matter, grey matter, and granular layers in the cerebellum, i.e. the corpus callosum or hippocampal formation. They are vividly imaged by means of mass spectrometry: e.g., see the cortex region: the yellow-greenish regions show co-localization of the green and red lipid species, while the purple-colored regions therein show co-localization of the red and blue lipid species.