Lipid testing is a cornerstone in atherosclerosis and cardiovascular disease (ASCVD) risk assessment. While there are several comprehensive clinical guidelines that focus on the primary prevention of ASCVD, guidance for the clinical lab remains limited. This causes significant variability in the way lipid testing is reported.
Here, we provide a general overview of recommendations from various clinical practice guidelines and briefly discuss considerations for lipid reporting in the clinical laboratory. More comprehensive evidence-based guidance on reporting, flagging, and interpretation is covered in the recently published Canadian Society of Clinical Chemists Harmonized Reference Interval Working Group (CSCC hRI-WG) recommendations.
What Should A Standard Lipid Profile Consist Of?
A standard lipid profile can provide the essential information needed to screen for dyslipidemia and establish CVD risk. A lipid panel should consist of total cholesterol, high density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), non-HDL-C, and triglycerides. This, along with relevant clinical and demographic information, allows for the estimation of ASCVD risk using a variety of endorsed tools, such as the Framingham Risk Score or the Pooled Cohort Equation. These tools are used by providers and patients to estimate the risk of a cardiovascular event in the next 10 years. LDL-C and non-HDL-C are used as treatment targets in patients being monitored over time. LDL-C is the primary treatment target; however, non-HDL-C is recommended as an alternative when triglycerides are elevated.
Apolipoprotein B (apo B) is another important test in ASCVD screening. These markers should be offered only as individual orderable tests and not a part of the standard panel. ApoB is recommended as an alternative treatment target and risk-enhancing biomarker by multiple guidelines. However, apoB is not currently endorsed as a routine screening measure because LDL-C and non-HDL-C are readily accessible on the standard report. Direct measurement of ApoB is particularly useful in situations where the calculated LDL-C or non-HDL-C result may not be accurate (e.g., genetic conditions of dyslipidemia).
The Emergence of Lp(a) in Clinical Guidelines
Recently, U.S., Canadian, and European cardiovascular societies have recommended Lp(a) testing to help identify patients at higher risk for ASCVD. As Lp(a) concentration is highly dependent on variations in the LPA gene, measurement is recommended only once in a person’s lifetime. Values ≥50 mg/dL (≥100 nmol/L) are associated with an increased risk of myocardial infarction and aortic valve stenosis; thus, it is recommended that these patients undergo earlier and more intensive health behavior modification and management.
What these clinical guidelines neglect is the significant heterogeneity in commercial Lp(a) assays. The apo(a) protein on Lp(a) exhibits significant variation in its size from person to person. The use of immunoassays with polyclonal antibodies against apo(a) will overlook the variation in apo(a) size and result in inaccurate Lp(a) results. Therefore, laboratories should select Lp(a) assays that are insensitive to apo(a) isoform size with calibrators traceable to the WHO/IFCC reference material. The most appropriate units of measurement for Lp(a) are nmol/L, as mass units (mg/dL) will be affected by the size of apo(a).
LDL-C Estimation: Farewell to Friedewald?
For decades, the Friedewald equation has been used to calculate LDL-C using results from components of the standard lipid panel. This equation is sufficiently accurate for normolipidemic patients; however, due to the nature of the formula (LDL-C = total cholesterol – HDL-C – (triglycerides/5)), hypertriglyceridemia can lead to the significant underestimation of LDL-C and can even produce negative (invalid) results. Due to the lack of direction from existing guidelines, a nonsensical result often gets reported and left to the interpretation of clinicians.
Novel estimation methods (e.g., Martin/Hopkins and Sampson/NIH equations) have been developed to improve LDL-C calculation, particularly in samples with triglycerides >400 mg/dL (4.5 mmol/L) or with low LDL-C. The 2019 American Heart Association/American College of Cardiology guidelines recommended the equation developed by Martin et al. (see suggested reading) as the preferred method for patients with low LDL-C.
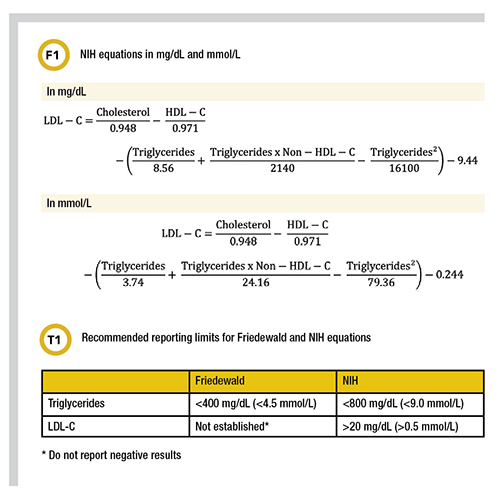
More recently, the NIH developed a novel equation (Figure 1) to calculate LDL-C in patients with triglycerides up to 800 mg/dL (9.0 mmol/L) with improved accuracy and without the pitfalls of the Friedewald equation. Others have validated this estimation method and have recommended reporting limits of >20 mg/dL (>0.5 mmol/L) for LDL-C and <800 mg/dL (9.0 mmol/L) for triglycerides (Table 1). An advantage of the NIH equation is that it can easily be implemented using the lipid parameters from the standard lipid panel. The equation below and is free to use.
To Flag Or Not to Flag? That Is the Question
In lipid testing, flagging a result is not as simple as setting a clinical cutoff, as target concentrations change based on clinical scenario. Lipid testing can be utilized for initial screening or for monitoring patients after the initiation of lipid-lowering therapy. However, due to the complexity of decision algorithms, it is challenging for a clinical laboratory to provide flags for each scenario.
There is agreement on which levels indicate when lipid-lowering treatment should be initiated—without any additional risk assessment. The evidence suggests that it is best to flag results lower than this cutoff to invite clinicians to assess if any additional risk factors might increase the likelihood of CVD. Further clarification on flagging limits and interpretive comments are included in the CSCC hRI-WG harmonized lipid reporting guidelines.
Food For Thought: Nonfasting Lipid Testing
The utility of nonfasting lipid testing continues to be promoted by many clinical practice guidelines. In short, for patients it is more convenient, encourages compliance, and yields accurate predictions for ASCVD risk, with the added advantage for the laboratory of reducing bottlenecks for sample collection and testing.
Although there is no consensus on triglyceride concentration that would require repeat testing in a fasted state, clinical scenarios warranting fasting lipid assessment include mainly patients with hypertriglyceridemia. Offering both fasting and nonfasting options (with the number of fasting hours recorded) is recommended for the most comprehensive ASCVD risk assessment.
Suggested Reading
Cegla J, France M, Marcovina SM, Neely RDG. Lp(a): When and how to measure it. Ann Clin Biochem 2021; doi: 10.1177/0004563220968473
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; doi: 10.1016/j.jacc.2018.11.002
Higgins V, Leiter LA, Delaney SR, Beriault DR. Validating the NIH LDL-C equation in a specialized lipid cohort: Does it add up? Clin Biochem. 2022; doi: 10.1016/j.clinbiochem.2021.10.003
Martin SS, Blaha MJ, Elshazly MB, Toth PP, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013; doi: 10.1001/jama.2013.280532
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2021; doi: 10.1016/j.cjca.2021.03.016
Sampson M, Ling C, Sun Q, Harb R, Ashmaig M, Warnick R, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol 2020; doi: 10.1001/jamacardio.2020.0013
White-Al Habeeb NMA, Higgins V, Venner AA, Bailey D, Beriault DR, Collier C, et al. Canadian Society of Clinical Chemists (CSCC) harmonized clinical laboratory lipid reporting recommendations based pm 2-21 Canadian Cardiovascular Society Lipid Guidelines. Can J Cardiol. 2022, doi: https://doi.org/10.1016/j.cjca.2022.03.019
Sarah Delaney, PhD, DABCC is a clinical biochemist at Unity Health Toronto and an Assistant Professor in the Department Laboratory Medicine and Pathobiology at the University of Toronto in Toronto, Ontario, Canada. +Email: [email protected]
Daniel Beriault, PhD, FCACB is a division head and clinical biochemist at Unity Health Toronto and an Assistant Professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto in Toronto, Ontario, Canada. +Email: [email protected]