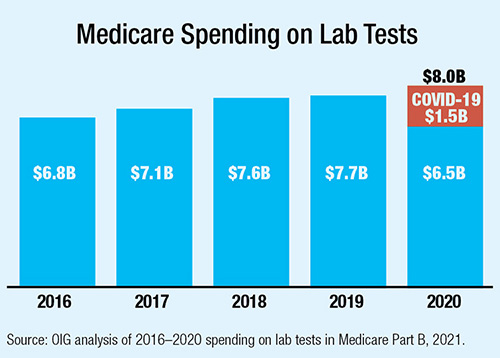
Medicare spent significantly more on clinical laboratory testing in 2020 as the COVID-19 pandemic drove demand for SARS-CoV-2 testing. But the data, released in a report from the Department of Health and Human Services Office of Inspector General (OIG), also reveals a decrease in non-COVID-19-related testing of more than $1 billion.
Overall spending increased 4%, from $7.7 billion in 2019 to $8.0 billion in 2020, with $1.5 billion in new spending on SARS-CoV-2 tests. Spending on rapid SARS-CoV-2 tests alone reached $1 billion. The report noted that while routine testing fell dramatically, part of the reduction in spending on other tests was due to reductions in payment rates required by the Protecting Access to Medicare Act of 2014. Since OIG began reporting this data in 2016, total spending increased each year by an average of 4.3% per year.
OIG did find that while non-COVID-19 testing recovered in the second half of 2020, the number of non-COVID-19 tests Medicare paid for during the full year still declined by 12%. Chemistry tests are the largest category by volume and spending, and their decline led the overall slump with a full 12% drop in volume, from 174 million in 2019 to 153 million in 2020.
OIG said that the decline in non-COVID-19 testing “raises questions about the potential impacts on beneficiary health … If Medicare beneficiaries delayed or avoided preventative healthcare services, they may not have received important tests, such as cancer screenings, that are medically necessary but not urgent. Research suggests that delays of such lab tests could have a long-lasting impact.”
New Standard for Opioid Withdrawal in Infants
The Department of Health and Human Services (HHS) and a group of clinicians and policy experts have published a standard clinical definition for opioid withdrawal in infants (J Pediatr 2021; doi: 10.1016/j.jpeds.2021.12.021). Notably, it also includes principles on bioethical uses for the definition, emphasizing that it is not meant to prove or imply harm and should not be used to assess child social welfare risk or status.
The number of mothers with opioid-related diagnoses documented at delivery increased approximately 130% from 2010 to 2017, HHS said. And infants with opioid exposure and withdrawal lack consistent diagnosis and care.
The new clinical criteria for diagnosis require the presence of two sets of clinical elements. The first includes in utero exposure to opioids with or without other psychotropic substances. It’s recommended this be collected via confidential maternal self-report or through toxicology testing with maternal informed consent. This should be combined with any two clinical signs: excessive crying, fragmented sleep, tremors, increased muscle tone, or gastrointestinal dysfunction.
The lack of a standard definition “has been a historical gap in the care of mothers and infants affected by opioid exposure, and created inconsistencies in diagnosing infants,” the report said.
Record Numbers Sign Up at Healthcare.gov
The Biden Administration released data showing that a record-breaking 14.5 million people signed up for healthcare coverage in 2022 through the federal marketplaces, including 5.8 million people who have newly gained coverage. This is about 20% higher than last year.
The American Rescue Plan Act (ARPA) lowered costs for most marketplace consumers: HealthCare.gov consumers saw their average monthly premium fall by 23% compared with the 2021 enrollment period that ended before the law passed.
It will be up to Congress whether to extend the subsidies from the ARPA past their expiration at the end of 2022. The Congressional Budget Office has estimated that the temporary subsidies under the ARPA would increase federal deficits by $34.2 billion. Without an extension, the average premium could double, according to analysis by the Kaiser Family Foundation.