Alternate specimen collection kits for genetic testing aren’t new—so-called spit kits and cheek swabs have been around for more than a decade (1). Although blood is often the preferred sample type for genetic testing due to the large amount of high-quality DNA that can be extracted from white blood cells, alternate sample types such as saliva and buccal cells can provide enough DNA to perform most genetic testing. Based on the clinical scenario, these samples may be easier to collect or preferred for testing to provide the most accurate results. For example, affected tissue may be preferred over a blood sample in a patient with a suspected mosaic condition, but a saliva or buccal sample may be preferred over blood in a patient who has had a bone marrow transplant.
Clinical laboratories require an order be matched to a physical sample that can be tracked throughout its lifecycle from collection to result (2). When a blood sample is collected for genetic testing, the clinic or laboratory will follow defined processes to get samples from the collection site to the reference laboratory. When an alternate sample, like saliva or buccal swab, is utilized for genetic testing, the patient may collect their sample remotely. With sample collection happening in a variety of locations, labs must create flexible logistics solutions for sample tracking to ensure proper handling and reporting of patient results.
Discovering Inspiration During the COVID-19 Pandemic
When the COVID-19 pandemic began, many outpatient clinics cancelled their in-person appointments and moved to virtual visits, and our pediatric tertiary care facility was no exception. With fewer patients attending their appointments in-person, and many caregivers not wanting to risk exposure by bringing their child into the lab for a blood draw, providers shifted to offering sample collection by alternate specimen kits, when appropriate.
Because of the complexities of order logistics and institutional billing, our laboratory team created new processes to support this increase in requests for alternate sample kits. This new process has supported patient access to genetic testing during an extended pandemic, and it also has allowed patients for whom collecting blood may have been challenging to access the genetic testing they need and deserve for their healthcare (3).
Our institution serves patients in a large geographical region (the states of Washington, Alaska, Montana, Idaho, and sometimes others), so collecting a blood sample for genetic testing is not always realistic, especially during the pandemic. The alternate specimen collection kit process greatly increased access for these remote patients. In addition, in 2020 our facility retired DNA banking and restricted use of the no-cost option to hold a sample for a short period of time for one desired genetic test. The option to collect samples for genetic testing with alternate specimen collection kits has therefore eased the burden of limited sample hold options for ordering clinicians.
Implementing Alternate Sample Collection Kits
Prior to the pandemic, our outpatient clinics primarily utilized alternate sample collection kits to collect parental comparator samples for trio-based genetic tests, such as exome sequencing. As our patients and providers moved to telehealth, the utilization of alternate sample collection kits increased. In the first 6 months of data tracking (April−September 2020), our laboratory team sent out 312 kits (around 52 kits per month). In the 6 months prior to this writing (April−September 2021), our laboratory team sent out 587 kits, or nearly 98 kits per month—a 188% year-over-year increase. With the increased request volume, we needed a standardized process to reliably handle these samples.
At our institution, a team of laboratory genetic counselors and genetic counseling assistants review all preauthorization requests for genetic testing. Once the preauthorization is in place, the ordering clinician enters the test order in the electronic medical record (EMR) and a sample can be collected for either in-house or sendout testing. To comply with our institutional billing processes, the sample must be routed through our hospital lab for processing prior to sending it to the intended reference lab for testing.
To align orders for genetic testing using alternate specimen collection kits with the standard order workflow, our laboratory team sends kits directly from our sendouts department to the patient’s home, instead of directly from the intended reference lab. This allows our laboratory team to include return shipping materials to route the collected sample back through our facility, both to align with institutional billing regulations and to assure the correct requisition is sent to the reference laboratory with the sample.
Our laboratory team created a kit handoff form for clinical teams to communicate their requests for alternate specimen kits. Requests are tracked and triaged by both the laboratory genetic counseling assistants and members of the lab sendouts team (Figure 1).
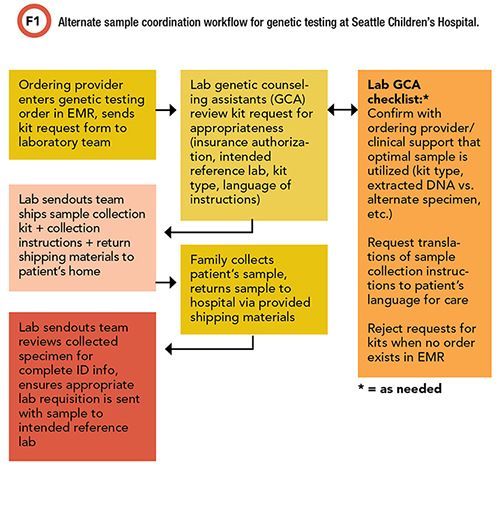
Refining Our Process
As our efforts to fulfill requests for alternate specimen collection kits progressed, it became clear that not everyone understood the types of collection kits available from reference laboratories used by our institution. For example, some laboratories offer buccal swabs and saliva collection kits, while others offer only one option or the other.
Further complicating things, not all saliva collection kits are suitable for all patients. Saliva kits can include an assisted collection method, which uses small sponges to absorb the saliva in the patient’s mouth. This is ideal for young pediatric patients and those with developmental differences that make spitting difficult. Other saliva kits require the patient to physically give the full sample directly into the collection tube.
To tackle this problem, our teams reviewed the indicated kit type to confirm that it was appropriate for the patient’s age and abilities and an accepted sample type by the performing reference lab. Additionally, many of our patients and families speak and read a language other than English. While most of the major kit manufacturers include translations of their collection instructions on their website, our laboratory team confirmed the patient’s language for care and modified our triage process to ensure that the appropriately translated instructions are included with the kit.
We also created an information page within our hospital’s online test catalog. This page consolidates the various sources of information related to the alternate specimen collection kits for genetic testing. The webpage includes a link to our kit request handoff form, as well as instructions for clinicians about the ordering process and background information on the clinical utility of buccal and saliva samples for genetic testing. We also added links to translated documents and collection instructions for each specific type of collection kit used by reference labs, as well as YouTube videos of the collection instructions that clinical teams can use to walk patients and families through the sample collection process. These resources have allowed more patients to successfully collect samples and complete genetic testing.
This alternate sample coordination process is continually evolving, but it’s more than just “kitting” around: Our new process for collecting and utilizing saliva samples and buccal swabs for genetic testing is truly patient-centered. We hope learning about our experience is useful for other facilities interested in implementing similar stewardship efforts to support patient access to genetic testing that is important and necessary for high quality care.
Clair Wittowski is a genetic counseling assistant at Seattle Children’s Hospital in Seattle. +Email: [email protected]
References
- Sun F, Reichenberger EJ. Saliva as a source of genomic DNA for genetic studies: review of current methods and applications. Oral Health Dent Manag 2014;13(2):217-22.
- MacMillan D, Lewandrowski E, Lewandrowski K. An analysis of reference laboratory (send out) testing: an 8-year experience in a large academic medical center. Clin Leadersh Manag Rev 2004;18(4):216-9
.
- Astion ML. Moving toward patient-centered laboratory stewardship. https://www.myadlm.org/cln/articles/2021/july/moving-toward-patient-centered-laboratory-stewardship. (Accessed November 16, 2021).