As in other disciplines, disruptive technology in laboratory medicine is often the brainchild of necessity. The focus on patient care drives both clinical laboratorians’ obsession with quality and their passion for ingenuity and invention.
Figuring out how to do something better often takes complete reimagining of a process to open space for the next generation of technology. The companies that participated in the AACC Disruptive Technology Award program at the 2020 AACC Annual Scientific Meeting in December illustrated this type of quest.
From designing diagnostic tests for babies instead of co-opting adult-ready assays, to using artificial intelligence to identify biomarkers for psychiatric disorders, to making tests for something as common as strep throat as easy and inexpensive as a pregnancy dipstick, Disruptive Technology Award finalists showed they are disrupting lab medicine for good.
Testing Babies at Baby Scale
Baebies impressed both the judges and AACC Annual Scientific Meeting attendees, winning the top prize in the competition as well as the Audience Choice Award, which was determined by audience voting.
The problem for patients the company wants to fix is common and simple: Babies need clinical laboratory testing as much as adults, but these tiny patients just don’t have a lot of blood, especially those born prematurely. Typically, babies have transfusions to make up for the volume of blood depleted through testing, but this raises the risk of infection and complications.
“Right now all devices that are being used for diagnostic testing for babies are typically intended for adults,” said Rama Sista, PhD, senior director of point-of-care products at Baebies. “Babies are not tiny adults. There’s just no platform out there that focuses on babies only.”
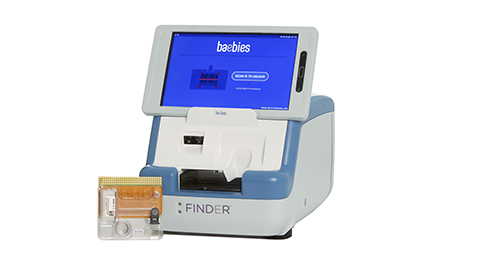
Baebies’ FINDER is an all-in-one diagnostic kit that tackles a common problem in treating the smallest patients. It uses digital microfluidics to move discrete droplets of fluid by electrical control of surface tension, or electrowetting, to perform bioassay protocols. This means the kit needs just 50 μL of blood to work. FINDER “has access to discrete droplets and can do pretty much anything you typically do in a laboratory,” said Sista. The platform works with multifunctional assay methods, including molecular, immunoassay, and chemistry, on the same cartridge. Results take 15 minutes.
The size of FINDER is a key part of the technology, too: It’s about 8 inches wide with a tablet interface, and is designed to be a bedside tool without requiring specialized laboratory knowledge or skills.
Sista said that the company’s priority is making testing easier for babies, but recognizes that FINDER also has utility in adult patients. The company plans to apply to the Food and Drug Administration (FDA) for an Emergency Use Authorization for a SARS-CoV-2 reverse transcription polymerase chain reaction test that provides results in 14 to 17 minutes. “We’re not inventing new assays,” said Sista. “All we’re doing is miniaturizing everything because of the capabilities of the technology.”
Molecular Testing, At Home
While the need for at-home SARS-CoV-2 testing has been a pressing focus of the IVD community, Sherlock Biosciences has been working to make more ordinary tests needed in non-pandemic times easier, cheaper, and feasible for patients to perform at home, similar to at-home pregnancy tests.
Sherlock’s INSPECTR platform, a finalist for the AACC Disruptive Technology Award, “enables molecular testing at the simplicity of use and price point of a simple dipstick immunoassay,” said William J. Blake, PhD, the company’s chief technology officer. The technology “combines the features of antigen tests that are low cost and simple with the accurate high specificity of sensitive molecular testing,” he added.
INSPECTR is a CRISPR-based diagnostic platform that combines nucleic acid preamplification with CRISPR-Cas enzymology for specific recognition of desired DNA or RNA sequences. This allows for multiplexed, portable, and ultra-sensitive detection of RNA or DNA from clinically relevant samples. Another unique aspect of the system is its use of freeze-dried synthetic gene networks.
The company is deploying the platform for SARS-CoV-2 testing. Sherlock has an FDA-authorized SAR-CoV-2 self-testing kit using the INSPECTR platform that returns results in about an hour. In December, the Bill & Melinda Gates Foundation awarded Sherlock a $5 million grant to advance the INSPECTR platform for SARS-CoV-2 testing, and the company plans to launch a home-based test by mid-2021.
Sherlock’s overall goal remains to make routine molecular testing simpler. Blake uses the example of a child who might have strep throat. Instead of a parent taking off from work and pulling the child out of school, a clinician could prescribe a test performed “in the comfort of the patient’s home with a technology that is as accurate as the most accurate molecular tests today,” he said. The INSPECTR technology is truly disruptive, Blake stressed, because the goal is to be “low cost and accessible to the individual, and not require instrumentation.”
Precision Diagnostics for Psychiatry
Right now, it’s not easy for psychiatrists to differentiate between bipolar disorder and depression because the symptoms are so similar. Patients typically go 7 years before getting an appropriate diagnosis of bipolar disorder, said Marianne Morini, business development manager at Alcediag. Moreover, 69% of those with the disease are initially misdiagnosed.
The results of a misdiagnosis can be catastrophic. Bipolar disorder is one of the 10 most disabling diseases, according to the World Health Organization. Being prescribed the wrong medication after a misdiagnosis could pose disastrous side effects. Bipolar disorder also presents a financial burden, both to patients seeking treatment and to society, with care costing $24 billion a year in the U.S. alone.
“We have treatments for bipolar disorder and depression, and people can be stabilized if they have the right diagnosis,” Morini said.
Alcediag’s EDIT-B, a finalist for the AACC Disruptive Technology Award, is the first blood-based diagnostic test for bipolar disorder—and for any psychiatric disease. The platform uses RNA sequencing and artificial intelligence to look at epigenetic biomarkers, specifically RNA site mutations, to determine “which combinations of those mutations on different sites of different biomarkers are significant characteristics of bipolar disorder,” Morini said.
EDIT-B sequences the DNA and calculates the level of editing in RNA; then an algorithm takes the sequencing data and creates a score that determines how likely the depressed person is to suffer from bipolar disorder. So far, the company says studies show EDIT-B is 87% accurate in this diagnosis.
Alcediag is currently in talks with U.S.-based laboratories to “put the test in a clinical laboratory setting,” said Morini, a step toward FDA authorization and broader dissemination. She knows those regulatory roads are long, but she’s optimistic about what EDIT-B can do, not just in diagnosing bipolar disorder but also other psychiatric conditions. Morini also believes that the technology could be used in other fields of medicine, including oncology. “We are at the beginning of the story,” she said.
Proposal submissions for AACC's 2021 Disruptive Technology Award are now being accepted until April 28, 2021. Learn more at https://meeting.myadlm.org/conference-program/disruptive-technology-award